Thermodynamics lecture 2
- 1. BITS Pil i Pilani Pilani Campus Lecture 2: P L t 2 Processes; Th Thermal equilibrium and l ilib i d Temperature; Phase behavior of pure substance
- 2. State Postulate • Phase – Spatially uniform (in chemical composition and physical properties), mechanically separable part of system • Homogeneous – single phase, else heterogeneous • Pure substance – one of unvarying chemical constitution • Simple, compressible substance – only form of work that of volume change, no magnetic, electrical, effects etc., Also we will normally ignore surface effects • Postulate – two intensive properties suffice to determine all others (ie., determine the equilibrium state) of a single phase, pure simple compressible substance. If i addition, h i l ibl b t If, in dditi the mass is known then so are all other extensive properties • Also applies to a mixture of fixed composition such as air in a single phase BITSPilani, Pilani Campus
- 3. Equilibrium surface • Properties are also called state functions • Set of all equilibrium states constitutes a surface in the space of independent intensive variables BITSPilani, Pilani Campus
- 4. Process • Process – system goes from state i to state f. In so doing, in general it will interact with the surroundings • Quasistatic process – intervening states are all equilibrium states – slow and controlled • Isobaric isochoric, isothermal processes Isobaric, isochoric •If intervening states not equilibrium states, then shown dashed • Cycle – Initial and final states are the same BITSPilani, Pilani Campus
- 5. Thermal Equilibrium • Diathermal material – One which allows two systems in contact across a rigid wall of such a material to influence g each other’s state, eg., copper. (It is a thermal conductor) • Adiabatic material – One which does not permit such an interaction as above when in the form of a rigid wall separating two systems, ie., it is a thermal insulator •S t Systems separated by a di th t db diathermal wall are i th l ll in thermal l contact, and will reach thermal equilibrium • Zeroth Law of Thermodynamics – If A and B are separately in thermal equilibrium with C, then A and B will be in thermal equilibrium with one another • An experiment with gases – equation of state BITSPilani, Pilani Campus
- 6. Temperature and Thermometry • States in thermal equilibrium with one another have the same temperature T • Equation of state: A relationship between P, v, and T, characteristic of a substance • T can be used as one of the variables t characterize th b d f th i bl to h t i the state, ie., v= v(P,T) • Thermometry: Such a relationship that enables one to determine the temperature from a measurement of a property for eg., height of mercury in capillary, resistance of a wire pressure of a fixed volume of a gas wire, • T also determines as we all know the direction in which heat transfer occurs, though we will introduce the concept of g p heat formally a little later in this course. BITSPilani, Pilani Campus
- 7. Pressure • Pressure: Normal force exerted by a fluid per unit area P = δFn/δA SI unit 1 Pascal (Pa) = 1 N/m2 1 bar = 105 Pa = 0.1MPa = 100kPa 1 bar = 105 Pa = 0 1MPa = 100kPa 1 atm = 101325 Pa = 101.325kPa 1 Torr = 1mm of Hg = 133.3224Pa g • Absolute Pressure and Gauge Pressure • Hydrostatic Pressure – due to a column of fluid of height h in gravitational field ∆P = ρgh is the pressure difference ρg p BITSPilani, Pilani Campus
- 8. ∫›∫›fl£ headings here Barometer¬†measuring¬† Manometer¬†measuring¬†pressure¬† p absolute¬†pressure relative¬†to¬†atmospheric¬†pressure p p BITSPilani, Pilani Campus
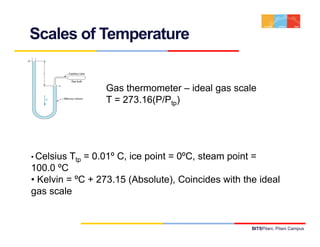
- 9. Scales of Temperature Gas thermometer – ideal gas scale T = 273.16(P/Ptp) • Celsius Ttp = 0.01º C, ice point = 0ºC, steam point = 100.0 ºCC • Kelvin = ºC + 273.15 (Absolute), Coincides with the ideal gas scale BITSPilani, Pilani Campus
- 10. Pure Substance Phase Behavior • Experiment with water at Constant Pressure: T-v behavior • Saturation Temperature – temperature at which liquid and vapor coexist at given P, ie., the boiling temperature • Saturation Pressure – pressure at which liquid and vapor coexist at given Y i th vapor pressure i Y, ie., the • The saturation T of water at 0.1 MPa is 99.6º C, and vice versa • At fixed pressure, the temperature does not change as long as the two phases coexist If heat is added the relative amount of vapor increases coexist. added, BITSPilani, Pilani Campus
- 11. Vapor Pressure • The vapor pressure of a pure liquid increases with increasing temperature • The vapor pressure has a unique value at a given temperature • The vapor pressure curve terminates at a critical point beyond which there is no distinction between liquid and vapor BITSPilani, Pilani Campus
- 12. T-v diagram for water BITSPilani, Pilani Campus