Thesis final copy
- 1. DRUG DISCRIMINATION FORAGING Discriminative Stimulus Effects of Nicotine during a Foraging Analysis Caitlan Byrnes Saint Anselm College November 2014 Acknowledgments
- 2. DRUG DISCRIMINATION FORAGING I would like to take this time to thank those who helped me progress and complete this experiment. I would like to especially thank Professor Troisi for aiding my studies and providing me with necessary knowledge and equipment. I would also like to take this time to thank Sam Dalhberg for caring for the laboratory animals! Abstract
- 3. DRUG DISCRIMINATION FORAGING Animal models of drug discrimination have served as a backbone for human research concerning addiction for centuries. Nicotine, a commonly used drug worldwide, can increase behaviors that result in a delivery of non-drug reinforcers. Consequently, nicotine has primary reinforcing effects and can strengthen operant behaviors that lead to a delivery of rewarding stimuli such as food or water (Bevins & Palmatier, 2004). The intent of the performed study was to define nicotine as a modulator for foraging behavior and then acquisition laboratory animals to associate a particular drug state with a reward (water). The study begins with some introductory knowledge essential for understanding and interpreting results by utilizing four subheadings; Conditioning: Operant and Pavlovian, Drug Discrimination, Foraging and Practical Applications of use. Throughout investigation different tandem sequences of nicotine and saline were expected to establish discriminative stimulus control over spatial learning for four rats, resulting in a decreased latency time during a foraging analysis. It was predicted that the rate of voluntary responses would become more frequent in an SD drug sequence because it occasions a response- reinforcer relationship by reinforcing a behavior under a particular stimulus. Although results did not hold significance between the independent and dependent variables, further research is suggested to investigate modulation of behaviors using interoceptive cues. Internal cues are critical to addiction and relapse behavior because internal or external stimuli may trigger a neurological response within the brain that then may signal drug onset cues which when eliminated might lead to more successful drug treatments. One theory for drug addiction is that if we change the context internally and externally we may decrease associations between the two which elicits relapse behaviors. Table Of Contents Acknowledgments ...................................................................................... Pg 2
- 4. DRUG DISCRIMINATION FORAGING Abstract ...................................................................................................... Pg 3 Introduction ...................................................................................................... Pg 6 Conditioning: Operant and Pavlovian …………………………………. Pg 6 Drug Discrimination.....................................................................………. Pg 7 Foraging.................................................................................................… Pg 9 Practical Applications.....................................................................……… Pg 9 Method ...................................................................................................... Pg 10 Subjects .......................................................................................... Pg 10 Aparatus .......................................................................................... Pg 10 Procedure .......................................................................................... Pg 11 Results ...................................................................................................... Pg 13 Discussion ...................................................................................................... Pg 15 Tables and Figures .......................................................................................... Pg 17 Table 1: “ Latency to first cap” ............................................................... Pg 17 Table 2: “Latency to last cap”................................................................. Pg 19 Figure 1: “Latency to first cap in seconds-rat 1” .................................... Pg 17 Figure 2: “Latency to first cap in seconds-rat 2” .................................... Pg 18 Figure 3: “Latency to first cap in seconds-rat 3” .................................... Pg 18 Figure 4: “Latency to first cap in seconds-rat 4” .................................... Pg 18 Figure 5: “Latency to last cap in seconds-rat 1” .................................... Pg 19 Figure 6: “Latency to last cap in seconds-rat 2” .................................... Pg 19 Figure 7: “Latency to last cap in seconds-rat 3” .................................... Pg 20 Figure 8: “Latency to last cap in seconds-rat 4” .................................... Pg 20
- 5. DRUG DISCRIMINATION FORAGING References ...................................................................................................... Pg 21 Appendices ...................................................................................................... Pg 23 Appendix A: “Morris water maze” ...................................................... Pg 23 Appendix B: “Syringes used” .................................................. Pg 24 Appendix C: “Water caps used” .................................................... Pg 25 Discriminative Stimulus Effects of Nicotine during a Foraging Analysis As intelligent beings, it is natural to seek understanding regarding human behavior. As a result, animal models have become a backbone attempting to parallel human addiction responses. Many studies attempt to mirror human addiction behavior such as drug foraging where one
- 6. DRUG DISCRIMINATION FORAGING physically goes through specified rituals to obtain a substance (Grund,1993). This is caused by an association made between an internal or external stimuli which then triggers a neurological response within the brain that may signal drug onset cues. When eliminated, an individual may be less likely to relapse resulting in more successful drug treatments. One theory for drug addiction is that if we change the context internally and externally we may decrease associations between the two which elicits relapse behaviors (Siegel, Shepard & Ramos, 2002.) Another theory of drug addiction is that our behaviors are driven by a series of learning processes in which we acquire associations and modify our performance in response to a stimulus. Conditioning: Operant and Pavlovian Classical conditioning, is a psychological learning process that develops through associations made between an environmental stimulus and an internal or naturally occurring stimulus (Kimble,1967). This theory of learning, first introduced by physiologist Ivan Pavlov, involves pairing a sensory event to something biologically important, yielding a response. Pavlov suggests that organisms are capable of tracking cues that predict natural recourses such as food, especially when under restricted conditions. In his study, “Pavlov’s Dogs” excitation occurs when a neutral stimulus with no associations to a behavior (NS) is placed before a naturally occurring reflex. From this research, psychologists have concluded that associative connections made between stimuli and the environment, help shape behavior. As a result, behavior is a reflexive social cue, eliciting a response when aroused by a specific stimuli (Mazur, 2002). Behaviorism is a branch of Psychological thought that attempts to quantify, train, and alter behavior through a modification process (Krantz, 2013). Operant conditioning, first coined by B.F Skinner, is the second psychological theory used to describe behavioral learning. It
- 7. DRUG DISCRIMINATION FORAGING proposed that an organism is capable of modifying its behavior by associating that specific behavior to a series of rewards or punishments (Mazur, 2002). One may acquire these associations through observational learning, language or rule governed schedules, or behavior modification which utilizes adverse or reinforcement stimuli. Skinner described his theory of learning by presenting a novel or spontaneous behavior and inhibiting or provoking that behaviors recurrence with positive/negative reinforcement or punishment. Neurologically speaking, this is due to an autonomic response in the peripheral nervous system which when conditioned (CR) increases the action potential along many motor neurons within the brain (Troisi, 2014). His research outlined the important relationship between internal and external stimuli and how external stimuli may be altered to modify an organisms behavior. Skinner’s contribution to learning theory and his work with animal models have crossed over into human studies concerning drug addiction and treatment/therapy options. Drug Discrimination Research involving drug discrimination in rodent models, propose that a drug may serve as an interoceptive cue suggesting the presence of a biologically important item such as food (Troisi, 2014). An Interoceptive cue is a sensory response originating inside the body which then may have an effect on behavior. In research on classical drug discrimination, nicotine functions effectively as an operant discriminative stimulus. A “discriminative stimulus” (SD) occasions a response-reinforcer relationship by reinforcing a behavior under a particular stimulus. An SD , can also serve as an “S.Delta” (SΔ) by signaling that a behavior has no consequence and will likely reoccur (e.g., Troisi, Dooley, Craig, 2013; Troisi, Bryant, Kane, 2012). As a result, it has been proposed that the function of a conditioned stimulus, or learned stimuli (CS) is not only to signal the probability of an unconditioned stimuli (US), but also to serve as a spatial cue to guide
- 8. DRUG DISCRIMINATION FORAGING the animal in the environment (Blum & Abbott, 1996). Prior work (Davidson, Aparicio and Rescorla, 1988) evidenced that operant discriminative stimuli and Pavlovian features function hierarchically. Nicotine, a commonly used drug worldwide, can increase behaviors that result in a delivery of non-drug reinforcers such as rewards or punishments. Consequently, nicotine has primary reinforcing effects and can strengthen operant behaviors that lead to a delivery of encouraging/rewarding stimuli such as food or water (Bevins & Palmatier, 2004). Hypothetically if this is true, nicotine would enhance responding for strong reinforcers and may increase motivation to obtain rewards associated with administration. Nicotine is also hypothesized to enhance sensory properties such as taste, touch, or smell. Stimuli paired with nicotine and administered after injections occur may have been altered by the drug and may increase the value of the reinforcer (Troisi, 2014). The value of the reinforcer is essential to behavior consistency. In human drug addiction studies, drug use is positive reinforcement and encourages behavior to reoccur because an operant response (self-administration) directly produces a reinforcing effect through administration. When resources become scarce or looses is no longer contingent, foraging comes into play to acquire the desired reward such as a drug/food. Foraging Foraging behaviors can be found in all organisms including humans. In the wild, foraging behaviors reveal an organisms sensitivity to a specific stimuli in its environment. Foraging can be considered an adaptive response to increase the likelihood of survival and is paralleled in addiction studies as the drug seeking behavior that encourages relapse. When resources become limited, behavior changes and the internal association between reduced reinforcement increases behavioral variability (Gharib, Gade, & Roberts, 2004; Stahlman, Roberts, & Blaisdell, 2010).
- 9. DRUG DISCRIMINATION FORAGING According to the optimal foraging theory, an animal is expected to enter into a given activity depending on associated costs and benefits (Arcis, 2003). Behavioral variation is critical to foraging due to a decrease in resources which then promotes the stereotyping of a ritual to become more successful during testing. In previous studies performed on primates, competition for resources have behavioral consequences in females (Koenig, 2002) resulting in a reduced number of offspring due to feeding competition. When a reward increases activity, extinction burst is more likely to occur and this is what we know as relapse. Extinction burst is a phenomena in which previously reinforced operant behaviors are no longer signaling the presence of a reward, but the behavior continues to persist despite the absence of a reward Harris, Pentel, & LeSage, 2007). In human addiction studies this encourages behavioral variability to find access to more reinforcers (drugs) which then spontaneously reinforces itself. Practical Applications Animal models act as a framework for human studies. It can be debated that many of our medical achievements stem from laboratory research involving vertebrate animals. They give psychologists a comparable organism to model human interactions involving molecules, cells, and tissue responses. Studies involving interoceptive cues and drug addiction in animal and human models suggest that operant contingencies involving a response-reinforcement interaction coincide with classical Pavlovian contingencies (Troisi, 2013). If foraging behaviors were measured while using the drug discrimination paradigm previously mentioned, laboratory animals would be expected to show a rate of responding which demonstrates a discrimination between a drug context in the SD condition which indicates food or a biologically important resource and a saline condition and no reward; SΔ. It is expected that
- 10. DRUG DISCRIMINATION FORAGING internal cues, such as nicotine modulate foraging behaviors in laboratory animals based on a reward/punishment system. Method Subjects Test subjects involved four male Sprague Dawley rats who had no previous training in a drug discrimination paradigm. The rats were not exposed to any drug internally or externally until the study began on 9/11/14. The rats were 5 months old, originating from a private breeder and were housed within the psychology department of a small liberal catholic college in the Northeast. The animals were cared for daily under strict guidelines from the animal welfare from order of the National Institute of Health (NIH) Committee. The subjects were housed two per cage (4x6”) and were kept at a free-feeding weight throughout the study but were limited to 15 minutes of water daily to keep thirst below satiation. Apparatus See Appendix A for complete visual of the “Morris Water Maze” (Morris et al. 1982). The Apparatus is used to test Spatial learning, place learning, cognitive maps and memory through observation of a rat’s spatial cues to locate a specific object. The apparatus is a round circular encasement about 5 feet wide and 4.5 feet tall. Inside the apparatus is sawdust with 3 specific “landmarks” such as wooden blocks to make quantifying behavior easier. Behavior as seen from an experimenters point of view is difficult to quantify based on random movements the subject makes. The blocks allow the researcher to determine how many times a subject returns to a specific spot with or without water. See Appendix B for image of a 10cc syringe which was used for all injections required by this study. Drug doses were based on daily weights and the drug consistency was a makeup of .08mgs of powdered Nicotine dissolved into a saline mixture. See
- 11. DRUG DISCRIMINATION FORAGING Appendix C for visual of “water cap” used to contain 2ml of water (10mls total, 5 caps used). The water caps were used to hold the reinforcer “water” under the SD drug condition depending on the rat. Procedure The present study involves testing the discriminative stimulus effects of two drug sequences (nicotine reinforced & saline not reinforced/ nicotine not reinforced&saline reinforced; NIC+ & SAL-/ NIC- & SAL+); SD which sets the occasion for responding predicting the presentation of water, and (nicotine not reinforced&saline reinforced/ nicotine reinforced&saline not reinforced)NIC- & SAL+/NIC+ & SAL-); SΔ which is not paired with the reinforcer and does not indicate a presentation of a reward (not predicting water). Four male Sprague Dawley rats were water restricted to 15 minutes daily, to increase the value of the reinforcer during testing. The study required the administration of counterbalanced drug conditions; nicotine according to body weight(0.3mg/kg) and saline depending on whether they were SD or SΔ conditions. For two rats, (rats 2 and 4) nicotine will function as an SD in which the presence of NIC was hypothesized to increase behaviors and indicate a reward (water). For the other two rats (1 and 3), conditions were counterbalanced and saline functioned as an SΔ which was not reinforced. Before injection, fill the syringes accordingly to weight allowing 12 minutes for the drug onset period to set in. Once administered through injection underneath the abdomen, the laboratory animals were placed separately into the Morris pen at the same start location every time (to keep context as controlled as possible). Within the pen were strategically placed water bowls or no reward/empty caps (depending on the condition) along with 3 checkmarks such as a wooden block to serve as landmarks within the apparatus. There were 5 caps containing 2ml of water each allowing the rat to obtain 10ml of water during one session. The landmarks provided the
- 12. DRUG DISCRIMINATION FORAGING primary investigator with a systematic application of quantifying the rat’s behavior. This was defined by the rats turning behaviors, locomotion, returning behaviors etc. Locomotion was defined by the movement or the ability of the subject to move from one place to another. Turning and returning behaviors were based on the quantity of times a subject returned to a checkpoint for investigation. For participants in the SΔ condition there was a 10minute foraging period in which behaviors were measured and compared to that of rats in the SD condition. Rats on test days were drugged or non-drugged but water (the reward) was not present within the apparatus. The rats were placed into the apparatus for a period of 5 minutes and were not rewarded to measure behavioral increases/changes/etc. Behavioral increases were measured using latency times on a stopwatch to determine how quickly a subject could locate a water cap under a particular condition. Results The intent of the performed study was to define nicotine as a modulator for foraging behavior and then acquisition the laboratory animals to associate a particular drug state with a reward (water). Throughout investigation different tandem sequences of nicotine and saline established discriminative stimulus control over spatial learning and as a result decreased latency times during the foraging analysis. It was predicted that the rate of voluntary responses would be higher in a sequence during the SD condition because it occasions a response-reinforcer relationship by reinforcing a behavior under a particular stimulus. The two dependent measures in the performed study were the latency in minutes to the first and the last water cap over a series of sessions. The independent variables were SD and SΔ drug conditions. Latency times were
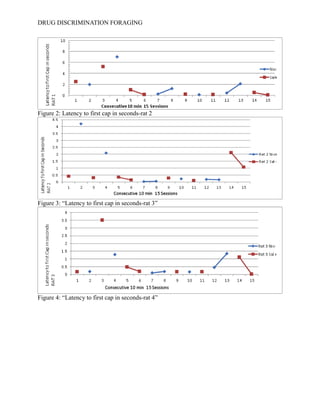
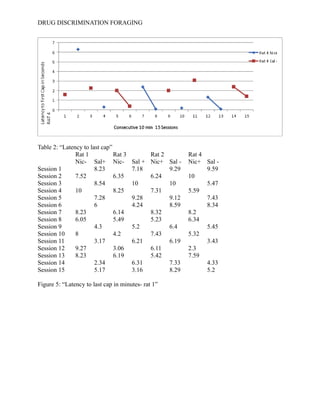
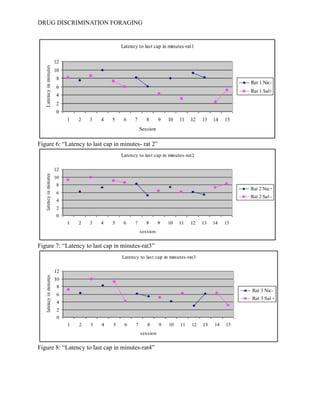
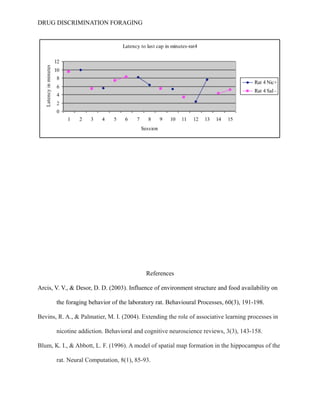
- 13. DRUG DISCRIMINATION FORAGING then evaluated to reveal whether shorter latency times were paired with rats receiving the SD condition, despite which drug was being reinforced during that interoceptive state. The data collected occurred over a period of 15 sessions and describe latency of each rat to the first cap found in the foraging space. -------See Table 1------Following the collected data is a figure which describes the relationship of rat one who received nicotine in the SΔ drug condition. ------See Figure 1-------- The figure illustrates rat 1’s latency to find the first cap in seconds. The table shows no significant correlation, ether positive or negative that would suggest that nicotine is modulating foraging behavior. During sessions 5-8, and 12-15 the figure demonstrates that rat one may have had a slight behavior modification. During session 5 to session 6, saline was reinforced and latency times decreased suggesting that an association between the drug and reward was made. Rat 2 received the opposite drug state in which nicotine was reinforced in the SD condition.--------See Figure 2--------The figure shows again no consistent pattern or trend to prove or disprove the original hypothesis, however between sessions 5-6 and 14-15 their is a decrease in response latency to the first cap. This may suggest that nicotine is modulating latency behaviors and is decreasing the amount of time it takes for a subject to find the first cap. In figure 3, rat 3 continues to show no significant pattern of response under the same condition as rat one. ------See Figure 3------ In Figure 4 the fourth rat’s latency to respond to the first cap is also shown. Rat 4 received the same internal drug state as rat 2.------See Figure 4-------The figure shows a peculiar relationship during sessions 5-8 because saline was not reinforced but responding decreased when it should have increased. Sessions 8-9 showed a significant decrease in responding under a rewarded condition (nic+) however, their was no consistent trend in behavioral responding.
- 14. DRUG DISCRIMINATION FORAGING Table 2 describes the latency in minutes of all four rats to retrieve the last cap of water within the apparatus. -----See Table 2-------- Figure one demonstrates a latency of responding for rat 1 that does not a significant trend. For rat 1 during sessions 7-9 , the primary observer detected a decrease in latency for responding during the SΔ condition when their should have been an increase. ----See Figure 5----- In later sessions, specifically sessions 14-15, rat one showed an increased latency for responding while under the SD condition when according to the hypothesis should have showed a decrease in responding. Rat 2 continued to show no significant trend of responding to the last cap. ----See Figure 6----- In Figure 6, rat 2 demonstrated a decreased latency under the SD condition in sessions 7-8, however responding increases around session 10 and continued to increase until session 15. Rat 3 had no trend in significant behavior. The latency for responding under a saline reinforced state fluctuated over the 15 sessions however, from sessions 3-6, the latency for finding all 5 water caps has the potential to start showing a negative trend (decreased latency times). This would agree with the hypothesis that the internal drug state, whether saline or nicotine may play a role in fluctuating behavioral foraging. During session 12, -----See Figure 7-------rat 3 increased latency time when saline was administered, again going against the original hypothesis. The final rat began to show significant results according to figure 8 by having a continuous decrease in latency, however rat 4 showed a decrease in latency under both conditions. ---See Figure 8---- Around session 11, behavioral variability increases and the laboratory animal increased its rate of responding when it should have continued to decrease. After consultation with several academic superiors, no inferential tests were performed. Because a t-test compares means between subjects and variables, and their are only four subjects and 2 independent and 2 dependent variables, an inferential analysis will
- 15. DRUG DISCRIMINATION FORAGING yield no extra information regarding significance of the study (Personal Consultation; P. Finn, 2014). Discussion Results gathered over 15 ten minute sessions, conclude that although nicotine has enhanced responding to strong reinforcers in past, it may not directly modulate foraging behaviors during an analysis. Previous work (Palmatier, O’Brien, & Hall, 2011), recognizes several of the confounding issues with this study. Their results suggested that the reinforcement enhancing effects of nicotine depend on conditioning history as well as behavioral motivation for the presented stimuli. The original hypothesis for the foraging analysis attempts to combine the enhancing effects of nicotine with a satiated stimulus to measure responding under each condition. Although results showed little significance, their were several methodological flaws worth noting that may have affected results. The laboratory animals were tested by the primary investigator daily over a period of about two months. The testing however, did not reoccur consistently at the same time every day. This may have affected results because of the animals feeding schedule. The animals were watered for 15 minutes daily around 530pm, and if testing was performed right before watering experimenters may be accidentally increasing the value of the reinforcer and results may be less symbolic of the expected foraging behavior. If testing were performed right after watering, the animals perhaps lost motivation for a devalued reward and as a result, had increased reaction times in the apparatus. Research for this study is ongoing and serves as a potential pilot for other similar drug studies. Further research should be done with more laboratory subjects, testing consistently at the same time every day over a period of several months. The results did not yield any significant trend, however nicotine should not be ruled out as an important internal cue that may modulate
- 16. DRUG DISCRIMINATION FORAGING behavior. From this study several human parallels can be made regarding drug treatment. If the study is replicated and significance is found, this information will lead to more efficient and faster drug treatments. This focuses on the internal issue of drug addiction, by taking away the internal cue that suggests the presentation of a drug, motivation to self-administer will diminish and drug seeking behavior will be inhibited. Tables and Figures Table 1: “Latency to first cap” Rat 1 Rat 3 Rat 2 Rat 4 Nic- Sal+ Nic- Sal + Nic+ Sal - Nic+ Sal - Session 1 2.49 0.18 0.4 1.57 Session 2 2 0.2 4.2 6.31 Session 3 5.2 3.5 0.3 2.24 Session 4 7 1.3 2.1 0.3 Session 5 1 0.48 0.35 2 Session 6 0.1 0.2 0.15 0.36 Session 7 0.26 0.1 0.04 2.38 Session 8 1.22 0.19 0.05 0.08 Session 9 0.17 0.18 0.28 2.01 Session 10 0.1 0.17 0.24 0.2 Session 11 0.09 0.18 0.1 3.07 Session 12 0.43 0.46 0.18 1.32 Session 13 2.11 1.36 0.17 0.1 Session 14 0.51 1.11 2.12 2.41 Session 15 0.08 0.02 1.06 1.39 Figure 1: Latency to first cap in seconds-rat 1
- 17. DRUG DISCRIMINATION FORAGING Figure 2: Latency to first cap in seconds-rat 2 Figure 3: “Latency to first cap in seconds-rat 3” Figure 4: “Latency to first cap in seconds-rat 4”
- 18. DRUG DISCRIMINATION FORAGING Table 2: “Latency to last cap” Rat 1 Rat 3 Rat 2 Rat 4 Nic- Sal+ Nic- Sal + Nic+ Sal - Nic+ Sal - Session 1 8.23 7.18 9.29 9.59 Session 2 7.52 6.35 6.24 10 Session 3 8.54 10 10 5.47 Session 4 10 8.25 7.31 5.59 Session 5 7.28 9.28 9.12 7.43 Session 6 6 4.24 8.59 8.34 Session 7 8.23 6.14 8.32 8.2 Session 8 6.05 5.49 5.23 6.34 Session 9 4.3 5.2 6.4 5.45 Session 10 8 4.2 7.43 5.32 Session 11 3.17 6.21 6.19 3.43 Session 12 9.27 3.06 6.11 2.3 Session 13 8.23 6.19 5.42 7.59 Session 14 2.34 6.31 7.33 4.33 Session 15 5.17 3.16 8.29 5.2 Figure 5: “Latency to last cap in minutes- rat 1”
- 19. DRUG DISCRIMINATION FORAGING Figure 6: “Latency to last cap in minutes- rat 2” Figure 7: “Latency to last cap in minutes-rat3” Figure 8: “Latency to last cap in minutes-rat4”
- 20. DRUG DISCRIMINATION FORAGING References Arcis, V. V., & Desor, D. D. (2003). Influence of environment structure and food availability on the foraging behavior of the laboratory rat. Behavioural Processes, 60(3), 191-198. Bevins, R. A., & Palmatier, M. I. (2004). Extending the role of associative learning processes in nicotine addiction. Behavioral and cognitive neuroscience reviews, 3(3), 143-158. Blum, K. I., & Abbott, L. F. (1996). A model of spatial map formation in the hippocampus of the rat. Neural Computation, 8(1), 85-93.
- 21. DRUG DISCRIMINATION FORAGING Gharib, A., Gade, C., & Roberts, S. (2004). Control of variation by reward probability. Journal of Experimental Psychology: Animal Behavior Processes, 30(4), 271. Grund, J-P.C.. (1993, March 16). Drug use as a social ritual. Instituut voor Verslavingsonderzoek, Rotterdam. Harris, A. C., Stepanov, I., Pentel, P. R., & LeSage, M. G. (2012). Delivery of nicotine in an extract of a smokeless tobacco product reduces its reinforcement-attenuating and discriminative stimulus effects in rats. Psychopharmacology, 220(3), 565-576. Kimble, G. A. (Ed.). (1967). Foundations of conditioning and learning. Appleton-Century-Crofts. Koenig, A. (2002). Competition for resources and its behavioral consequences among female primates. International Journal of Primatology, 23(4), 759-783. Krantz, D. L. (1971), THE SEPARATE WORLDS OF OPERANT AND NON-OPERANT PSYCHOLOGY. Journal of Applied Behavior Analysis, 4: 61–70. Mazur, James E. (2002) Learning and behavior (5th ed.). Upper Saddle River, NJ, US: Prentice Hall/Pearson Education. Morris, R.G.M. (1982). Spatial localization does not require the presence of local cues. Learning and Motivation, 12, 239-260. Palmatier, M. I., O’Brien, L. C., & Hall, M. J. (2012). The role of conditioning history and reinforcer strength in the reinforcement enhancing effects of nicotine in rats. Psychopharmacology, 219(4), 1119-1131. Stahlman, W., & Blaisdell, A. P. (2011). Reward probability and the variability of foraging behavior in rats. International Journal Of Comparative Psychology, 24(2), 168-176.
- 22. DRUG DISCRIMINATION FORAGING Troisi, J. R., II, Dooley, T, II, & Craig, E. (2013). The Discriminative stimulus effects of a nicotine-ethanol compound in rats: extinction with the parts differs from the whole. Behavioral Neuroscience, 127, 899-912. Troisi, J. R., II (2013). Perhaps More Consideration of Pavlovian-Operant Interaction May Improve the Clinical Efficacy of Behaviorally Based Drug Treatment Programs. The Psychological Record, 63, The Psychological Record, 63(4), 863-893. Troisi, J.R., II, Bryant, E., & Kane, J. (2012). Extinction of the discriminative stimulus effects of nicotine with a devalued reinforcer: Reinstatement following revaluation. The Psychological Record, 62, 707-718. Siegel, Shepard; Ramos, Barbara M. (2002). Applying laboratory research: Drug anticipation and the treatment of drug addiction. Experimental and Clinical Psychopharmacology, Vol 10 (3), Aug 2002, 162-183. Appendices Appendix A: Morris Water Maze (Morris et al, 1982)
- 23. DRUG DISCRIMINATION FORAGING Appendix B. 10cc Syringe used for injections