Mid-Study Transition of a Global Study: Migration from Paper TMF to eTMF
- 1. Mid-Study Transition of a Global Study: Migration from Paper TMF to eTMF ASHLEY SMITH, LIFE SCIENCES SOLUTIONS, PROJECT MANAGER TransPerfect- Trial Interactive OCTOBER 23, 2013
- 2. AGENDA ŌĆó Scope of Project ŌĆó Initial Steps ŌĆó Execution ŌĆó Challenges & Resolution
- 3. CLIENT NARRATIVE Client: Major Pharmaceutical Company conducting a Phase III study in 30 different countries ŌĆó Initially started using only one CRO as primary TMF holder ŌĆó Planned to expand to additional countries around the world ŌĆó Hired three additional CROs regionally ŌĆó Initiated the transfer of TMF during the study start up process Site Activation ŌĆó Goal of 400 sites world wide ŌĆó At the point of transfer 120 sites were active within 30 countries
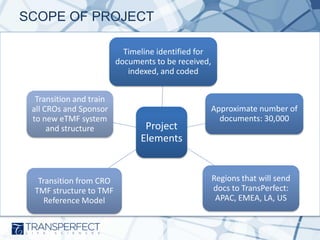
- 4. SCOPE OF PROJECT Timeline identified for documents to be received, indexed, and coded Transition and train all CROs and Sponsor to new eTMF system and structure Transition from CRO TMF structure to TMF Reference Model Project Elements Approximate number of documents: 30,000 Regions that will send docs to TransPerfect: APAC, EMEA, LA, US
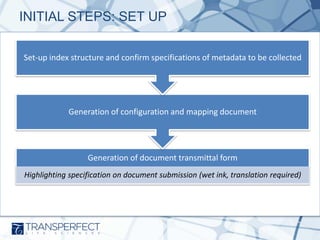
- 6. INITIAL STEPS: SET UP Set-up index structure and confirm specifications of metadata to be collected Generation of configuration and mapping document Generation of document transmittal form Highlighting specification on document submission (wet ink, translation required)
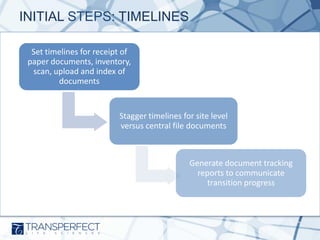
- 7. INITIAL STEPS: TIMELINES Set timelines for receipt of paper documents, inventory, scan, upload and index of documents Stagger timelines for site level versus central file documents Generate document tracking reports to communicate transition progress
- 8. PROCESS EXECUTION INDEXING ŌĆó Document transitional process of previous index structure to new index structure and share with client ŌĆó Mapping changes from the old index structure to the new REPORTING ŌĆó Develop reporting mechanism for illegible documents and documents that need to be resubmitted ŌĆó Standardization of receipt documentation for multiple submitters
- 9. EXECUTION ŌĆó Track priority countries and priority site document ŌĆó Inventory, assign document ID, scan, and code document ŌĆó Streamlined indexing operation in the US and UK ŌĆó Streamlined coding process in India, US, and UK
- 10. EXECUTION: TIMELINE ŌĆó Documents received: March 24,018 February 14,457 January 6,572 April 35,824
- 11. EXECUTION ŌĆó All documents coded and indexed by end of May 2013 ŌĆó Initiate a reconciliation process with paper files to ensure accuracy and completeness of paper files ŌĆó Reconciliation set to be complete by November 2013
- 12. CHALLENGES ŌĆó Multiple languages ŌĆó Resolution: Translation ŌĆó Upgrade: Inline translation of documents during coding process to increase efficiency
- 13. CHALLENGES ŌĆó Multiple changes to the index structure ŌĆō the site file and country level filing structure ŌĆó Resolution: File sign off on document mapping
- 14. SUMMARY ŌĆó ŌĆó Reduction in paper documents sent to eTMF Increased ability for reporting across all countries ŌĆó Increased visibility on study metrics
- 15. CONTACT INFORMATION Ashley Smith, PM Life Sciences Solutions TransPerfect 3 Park Ave New York, NY 212.689.5555
- 16. THANK YOU!