Tptz final
- 1. Under the guidance of Sri B. Thangabalan Sir M. Pharm (Ph.D) By K. Sampath Kumar, Y11MPH18060, I/II M.Pharm, 2nd Semester, Pharmaceutical Analysis, 1 Sims college of Pharmacy.
- 2. TPTZ Reagent N N N N N N Chemical name : 2, 4, 6, - Tripyridyl -s- triazine Molecular formula : C18H12N6 Molecular weight : 312.33 Melting point : 247-2490c Solubility 2 : Methanol 100mg/ml
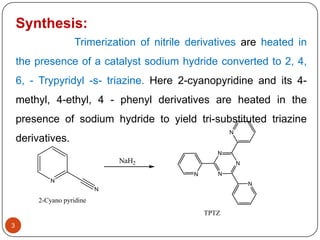
- 3. Synthesis: Trimerization of nitrile derivatives are heated in the presence of a catalyst sodium hydride converted to 2, 4, 6, - Trypyridyl -s- triazine. Here 2-cyanopyridine and its 4- methyl, 4-ethyl, 4 - phenyl derivatives are heated in the presence of sodium hydride to yield tri-substituted triazine N derivatives. N NaH2 N N N N N N 2-Cyano pyridine TPTZ 3
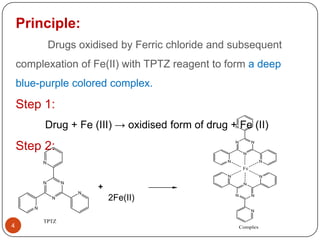
- 4. Principle: Drugs oxidised by Ferric chloride and subsequent complexation of Fe(II) with TPTZ reagent to form a deep blue-purple colored complex. Step 1: Drug + Fe (III) â oxidised form of drug + Fe (II) N N N Step 2: N N N N Fe N N N N N + N N N N 2Fe(II) N N TPTZ 4 Complex
- 5. Reagents preparation: TPTZ solution: About 330 mg of 2, 4, 6-tripyridyl-s-triazine was accurately weighed and dissolved in 100ml of methanol. FeCl3 stock solution: 162 mg of anhydrous FeCl3 in 100 ml of distilled water. 33.3 ml of above stock solution was further diluted to 100 ml with water. 50 ml of above stock solution was further diluted to 100 ml with water. Orthophosphoric acid: 5
- 6. 1. Estimation of Ethacridine lactate: Ethacridine Lactate with 2,4,6-tripyridyl-s-triazine in the presence of Fe(III) to form coloured chromophores which can be estimated at absorption 540nm respectively. 6
- 7. Procedure: 1000Ξg/ml standard solution 60 to 260 Ξg/ml in 10ml flasks 1 ml of 0.003 M ferric chloride + 1 ml of TPTZ solution gently boiled for 15 min cooled to room temperature and 2 ml of Ortho Phosphoric Acid Volume made up with distilled water 7
- 8. 2. Estimation of Vitamin-E1: It was based on the reduction of ferric ion to ferrous ion when Vitamin-E reacted with TPTZ forms color chromogen which can be estimated at 600nm. 8
- 9. Procedure: 200 mg/100ml in methanol 0.23, 0.3, 0.75, and 1.0 ml. of stock standard in 100ml flasks (0.5-2mg/ml) Volume made up to 100ml with methanol Absorbance measured at 600nm 9
- 10. 3. Estimation of N-Acetyl-L-cysteine O H N HO O HS This method is based on the coupled redox- complexation reaction between N-Acetyl-L-cysteine and Fe (III) uses 2, 4, 6- tripyridyl-s-triazine (TPTZ) as the chromogenic reagent. 10
- 11. 4. Estimation of n-(2-mercaptopropionyl)- glycine: This method is based on the coupled redox complexation reaction between N-(2- Mercaptopropionyl) glycine and Fe (III) uses 2, 4, 6- tripyridyl-s-triazine (TPTZ) as the chromogenic reagent. 11
- 12. Mechanism: Step I: In the first (redox) step of the reaction N-Acetyl-L-cysteine or N- (2-Mercaptopropionyl) glycine reduces Fe (III) to Fe (II) whereas RSH molecules themselves oxidize to thiyl radicals RS which combine to form the disulfide RSSR. Step II: In the second step of the reaction formed Fe(II) is immediately complexed by 2 molecules of TPTZ to form the deep-blue coloured, highly stable Fe(TPTZ)2 2+ complex which absorbs light at Îŧmax at 593 nm. 12
- 13. Procedure: 20mL of pH 3.6 acetate buffer in 25 volumetric flasks 1.25ml of 10 mM Fe (III) + 1.25ml of 10mM TPTZ + 1.0mL of N-Acetyl-L-cysteine or N-(2- Mercaptopropionyl) glycine solutions Volume made up with distilled water Kept aside for 30mins at RT Absorbance was measured at Îŧmax 593nm against a blank 13
- 14. 5. ESTIMATION OF URIC ACID: O H N NH O N H N O H uric acid One mole of uric acid reduces four moles of ferric ions to the ferrous form. 2, 4, 6 - Trypyridyl-s-triazine (TPTZ) reacts with the ferrous ions to form a blue colored complex which absorbs strongly at 590 nm. 14
- 15. Procedure: 1 part TPTZ REAGENT + 10 parts FERRIC - BUFFER REAGENT.(working standard) Dispense 2.00 ml of Working Reagent into tubes labeled: Reagent Blank, Calibrator, Control, Sample 1, etc. Place 0.02 ml Specimen into the appropriately labeled tube. Use deionized water as specimen for Reagent Blank. Mix well. kept aside for 15min Adjust instrument to zero absorbance at 590 nm using Reagent Blank. 15
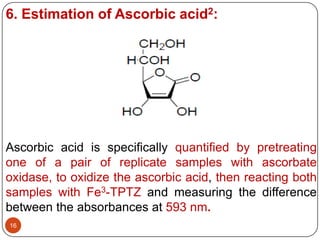
- 16. 6. Estimation of Ascorbic acid2: Ascorbic acid is specifically quantified by pretreating one of a pair of replicate samples with ascorbate oxidase, to oxidize the ascorbic acid, then reacting both samples with Fe3-TPTZ and measuring the difference between the absorbances at 593 nm. 16
- 17. 17
- 18. References: 1. Robert G. Martinek Method for the Determination of Vitamin E (Total Tocopherols) in Serum, Vol. 10, No. 12, 1964 Page No. 1078-1086. 2. Tsan Z. Liu, Nancy Chin, Michael D. Kiser, and William N. Bigler Specific Spectro photometry of Ascorbic Acid in Serum or Plasma by Use of Ascorbate Oxidase Vol. 28, No. 11, 1982 Page No: 2225-2228. 18
- 19. Itâs time for queries Donât worry I will receive it as a suggestionâĶ! Thank uâĶ.. K. Sampath Kumar 19