TR stages
- 2. • • There is a misconception about "Translational Research" terminology. I realized that terms used interchangeably but incorrectly as I was sharing my experience with people about my next opportunity.
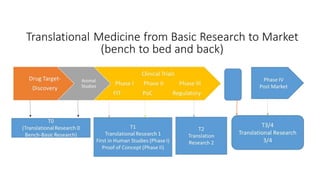
- 3. Translational Research (TR) from bench to bedside • Translational Research 0 (TR0): Wet lab-bench work, drug discovery and Animal Studies (PRECLINICAL STUDY- Early Development) • TR1: First in Human Studies (Phase 1) and Proof of concept Study (Phase2), Even though they are in human but not proven to be use to in human • TR2: Phase 3, example Drug Efficacy during development of a drug • TR3: Regulatory Studies required by regulatory bodies like FDA. The results from the Phase 3 studies used. • TR4: Phase 4, Surveillance-Post-marketing Studies
- 4. Technology Transfer to Improve Human Health Novel technologies potential to improve human health integrating scientific development technologies and conducting clinical studies. * Development phases classified in 5 technologies: 1- Drug 2- Medical Device 3- Biologics 4- Biomarker Analysis Assays 5- Imaging Procedures
- 5. What is PK and PD: How do they relate to each other? Pharmakokinetics (PK): • What the human body does to the body Pharmacodynamics (PD): • What a drug does to the human body
- 6. Objectives of a Clinical Trial We may have 3 Types of Clinical Trial Objectives: 1. Ideal Health/ Primordial Intervention/Healthy 2. Primary Prevention/ Risk Factors/Acute 3. Secondary Prevention/ Disease to treat disease/Chronic