Tricyclic antidepressants
- 2. яВЧ The TCAs are structurally related to each other and, consequently, possess related biological properties that can be summarized as characteristic of the group. яВЧ The TCAs are extremely lipophilic and, accordingly, very highly tissue bound outside the CNS. Because they have anticholinergic and noradrenergic effects, both central and peripheral side effects are often unpleasant and sometimes dangerous.
- 3. яВЧ Products: Imipramine Hydrochloride - It is the lead compound of the TCAs. It is also a close relative of the antipsychotic phenothiazines. - It has weaker D2 postsynaptic blocking activity than promazine and mainly affects amines via the transporters.
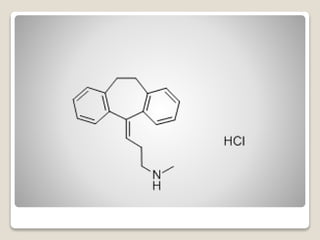
- 5. Desipramine Hydrochloride - Among tricyclics, desipramine would be considered when few anticholinergic effects or a low level of sedation are important.
- 6. яВЧ Norpramin (Desipramine Hydrochloride, USP) is an antidepressant drug of the tricyclic type, and is chemically: 5H-Dibenz[b╞Т]azepine-5- propanamine,10,11-dihydro-N-methyl-, monohydrochloride.
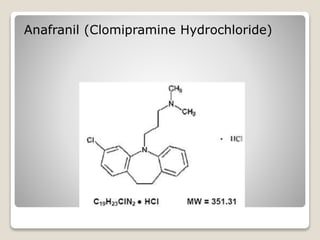
- 7. Clomipramine Hydrochloride - It is an up to 50 times as potent as imipramine in some bioassays. - This does not imply clinical superiority, but it might be informative about tricyclic and, possibly, other reuptake inhibitors.
- 9. яВЧ Amitriptyline Hydrochloride - It is one of the most anticholinergic and sedative of TCAs. Because it lacks the ring- electron-enriching nitrogen atom of imipramine.
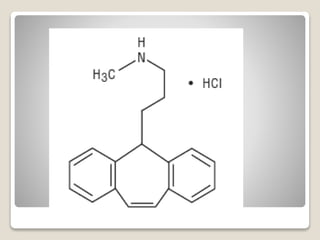
- 11. яВЧ Nortriptyline Hydrochloride - Nortriptyline is a selective NE transporter inhibitor. - Metabolic inactivation are like those of amitriptyline.
- 13. яВЧ Protriptyline Hydrochloride - Protriptyline is a structural isomer of nortriptyline. - Inactivation can be expected to involve the relatively localized double bond.
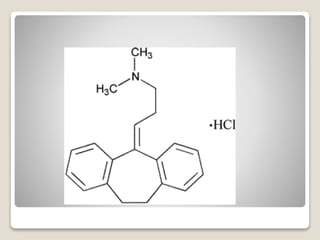
- 15. яВЧ Trimipramine Maleate - It is used as the racemic mixture. - Biological properties reported resemble those of imipramine
- 16. яВЧ Doxepin Hydrochloride - It is an oxa congener of amitriptyline, as can be seen in itтАЩs structure.
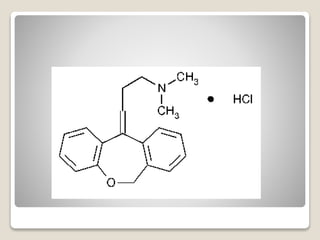
- 18. яВЧ Maprotiline Hydrochloride - It is sometimes described as a tetracyclic rather than a tricyclic antidepressant. - The compound is not strongly anticholinergic and has stimulant properties. It can have effects on the cardiovascular system.
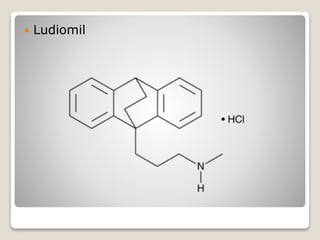
- 19. яВЧ Ludiomil
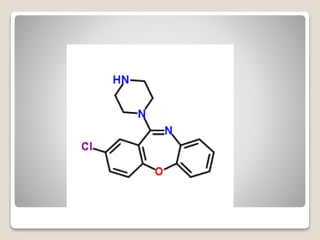
- 20. яВЧ Amoxapine - The N-methyl-substituted relative of amoxapine is the antipsychotic loxapine (Loxitane). - The 8-hydroxy metabolite of amoxapine is reportedly active as an antidepressant and as D2 receptor blocker.
- 23. яВЧ Structurally, the SSRIs differ from the tricyclics, in that the tricyclic system has been taken apart in the center. яВЧ Many of the dimethylamino tricyclics are, in fact, SSRIs. Because they are extensively N-demethylated in vivo to compounds, which are usually SNERIs, however, the overall effect is not selective.
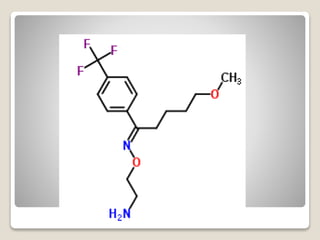
- 24. яВЧ Fluoxetine - In Fluoxetine (Prozac), protonated in vivo, the protonated amino group can H-bond to the ether oxygen electrons, which can generate the Beta-arylamino-like group, with the other aryl serving as the characteristic тАШтАЩextraтАЩтАЩ aryl.
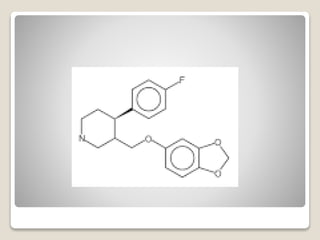
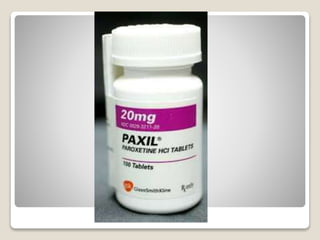
- 27. яВЧ Paroxetine - It is an effective antidepressant and anxiolytic.
- 30. яВЧ Sertraline - Inspection of sertraline (Zoloft) reveals the pharmacophore for SERT inhibition.. - The substituents also predict tropism for a 5-HT system.
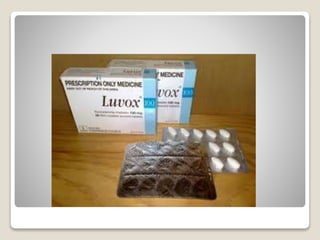
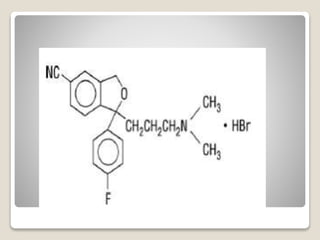
- 33. яВЧ Fluvoxamine - The E-isomer of fluvoxamine (Luvox) can fold after protonation to the Beta- arylamine-like grouping.
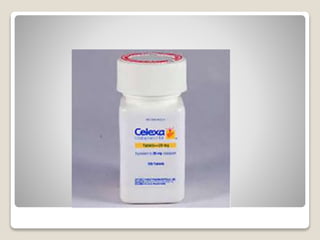
- 36. яВЧ Citalopram - Citalopram (Celexa) is a racemic mixture and is very SERT selective. - The N-monodemethylated compound is slightly less potent but is as selective.
- 39. яВЧ Selective Norepinephrine Reuptake Inhibitors
- 40. яВЧ The discussion of fluoxetine opened the subject SNERIs. That is, movement of the para substituent of fluoxetine to an ortho position produces SNERI.
- 41. яВЧ Nisoxetine - Nisoxetine is a SNERI and is an antidepressant. Most activity resides in the Beta-isomer.
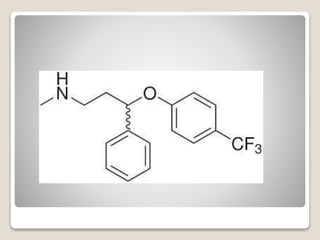
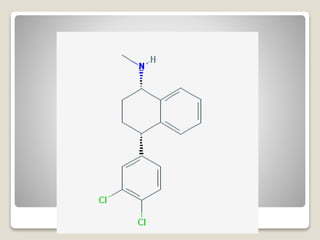
- 43. яВЧ Reboxetine - It is claimed to be superior to fluoxetine in severe depression. - They, of course, have typical characteristic TCA side effects but lower anticholinergic and H1-antihistaminic (sedative) effects than dimethyl compounds.





![яВЧ Norpramin (Desipramine Hydrochloride, USP) is
an antidepressant drug of the tricyclic type, and
is chemically: 5H-Dibenz[b╞Т]azepine-5-
propanamine,10,11-dihydro-N-methyl-,
monohydrochloride.](https://image.slidesharecdn.com/tricyclicantidepressants-150215221538-conversion-gate01/85/Tricyclic-antidepressants-6-320.jpg)