Troubleshooting Of Compression Issues with complete Solution
- 1. Copyright ┬® Keva Pharmaceuticals TROUBLESHOOTING OF COMPRESSION ISSUES Presenter: Suraj Kumar Sah 25th July , 2023 Keva Pharmaceuticals Pvt. Ltd., Ratnanagar-16, Chitwan 1
- 2. Copyright ┬® Keva Pharmaceuticals Before We Start ’üČWhat is Compression? ’üČCompression Machines at Keva PharmaceuticalsŌĆ” ’üČFactors affecting compressionŌĆ” ’üČProblems faced during compressionŌĆ” ’üČImpacts of compressionŌĆ” 2
- 3. Copyright ┬® Keva Pharmaceuticals Outline 1.Introduction 2.Factor affecting compression 3.Problem faced during compression 4.Impact of compression 5.Conclusion 6.References 7.Q & A 3
- 4. Copyright ┬® Keva Pharmaceuticals Introduction ’üČCompression: According to USP When we apply any force to powder bed then bulk volume will be reduced, known as Compression. ’āśThe reduction in the bulk volume of a material as a result of the removal of void space by applied pressure. ’üČCONSOLIDATION: ’āśInvolves an increase in the mechanical strength of a material resulting from particle-particle interactions. ’üČCOMPACTION: ’āśThe compression and consolidation of granules due to an applied force, known as Compaction. 4
- 5. Copyright ┬® Keva Pharmaceuticals Factor Affecting Compression ’āśSurface Properties ’āśPorosity ’āśFlow Properties ’āśCompression Force ’āśCompression Speed ’āśMoisture Content 5
- 6. Copyright ┬® Keva Pharmaceuticals ŌĆó 1. Capping ŌĆó 2. Lamination ŌĆó 3. Cracking ŌĆó 4. Chipping ŌĆó 5. Sticking ŌĆó 6. Picking ŌĆó 7. Mottling ŌĆó 8. Double Impression ŌĆó 9. Weight Variation ŌĆó 10. Hardness Variation ŌĆó 11. Friability PROBLEM FACED DURING COMPRESSION 6
- 7. Copyright ┬® Keva Pharmaceuticals Capping 7 ’üČCauses: ’āśDue to the air entrapment during compression. ’āśLow moisture in granules. ’āśDeep concave punches. ’üČRemedies/Solution: ’āśPrecompression, Reducing the final compression pressure. ’āśDry the granules properly. ’āśUse flat punches. Partial or complete separation of top or bottom crown of tablet.
- 8. Copyright ┬® Keva Pharmaceuticals Lamination Separation of a tablet in to two or more distinct horizontal layers. Causes: ŌĆó Too much compression pressure. ŌĆó Unwanted dried granules. Remedies: ’āśAccurate compression force should be apply to form tablet. ’āśAdd hygroscopic substances certain % of moisture by e.g. Sorbitol, Methylcellulose, PEG. 8
- 9. Copyright ┬® Keva Pharmaceuticals Cracking ŌĆó Small, fine cracks observed on the upper and lower central surface of tablets. ’üČReason: ’āś Deep concave punches. ’āśToo dry granules. ’üČRemedies: ’āśUsing Flat punches. ’āśMoisten the granules properly. 9
- 10. Copyright ┬® Keva Pharmaceuticals Chipping ŌĆó Breaking of tablet edges, while the tablet leaves the press or during subsequent handling. ŌĆó Reason: ’āśIncorrect machine settings/ Less Binder Remedies: ŌĆó The Causes and Remedies of chipping related to ŌĆśFormulationŌĆÖ 10 Sr. No. CAUSES REMEDIES 1. Machine RPM too fast Reduce machine speed 2. Too dry granules Increase the moisture content of granules. 3. Scratch/uneven Surface Smooth punch 10
- 11. Copyright ┬® Keva Pharmaceuticals Sticking ’āśSticking is the problem where the material adhere to die wall during tablet compression. ’āśReason: Fig: Sticking on punch Face. ’é¦ Pressure applied is higher. ’é¦ Excessive moisture in granules. ’é¦ Rough die surface. ’üČRemedies: ’āśDecrease the pressure to optimum. ’āś Proper drying of granules. ’āśAdding glidant and lubricants. 11
- 12. Copyright ┬® Keva Pharmaceuticals Picking 12 Material adhere to punch faces ’āś Picking is the term used when a small amount of material from a tablet is sticking to and being removed off from the tablet surface by a punch face.This occurs predominantly in upper faces of the punches.
- 13. Copyright ┬® Keva Pharmaceuticals Picking CAUSES ’āśExcessive moisture in granules ’āśImproper lubrication ’āśLow melting point substances added ’āśRough or scratched punch faces REMEDIES ’āśDrying of granules ’āśIncreasing lubrication ’āśUse high melting point lubricants ’āśPlatting of punch faces by - Chromium 13
- 14. Copyright ┬® Keva Pharmaceuticals Mottling ŌĆó Mottling is the term used to describe an unequal distribution of color on a tablet. ’āśCauses : ŌĆó Different colors of excipients or a drug ŌĆó Migration of dyes to the surface of granules during drying ŌĆó Improper mixing of color ’üČRemedies: ŌĆó Change the solvent system (binding system) ŌĆó Reduce the drying temperature ŌĆó Mix properly and reduce the size to prevent the mottling. 14
- 15. Copyright ┬® Keva Pharmaceuticals Double Impression ’āś Due to free rotation of the punches, having some engraving on their faces. ’é¦ Reasons For Double impression: ’āśUncontrolled rotation of either upper punch or lower punch during ejection of a tablet. ’é¦ Solution/Remedies: ŌĆó Use of keyed punches. ŌĆó Use of Anti-Turning Devices. 15
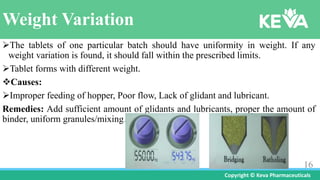
- 16. Copyright ┬® Keva Pharmaceuticals Weight Variation ’āśThe tablets of one particular batch should have uniformity in weight. If any weight variation is found, it should fall within the prescribed limits. ’āśTablet forms with different weight. ’üČCauses: ’āśImproper feeding of hopper, Poor flow, Lack of glidant and lubricant. Remedies: Add sufficient amount of glidants and lubricants, proper the amount of binder, uniform granules/mixing. 16
- 17. Copyright ┬® Keva Pharmaceuticals Hardness Variation ŌĆó Tablets having different hardness value. Generally, as granules size increases, tablets are found to show increased weight variation, and same occur with the hardness. ’üČCauses : ŌĆó Improper binder concentration and uniformity. ŌĆó Room temperature and RH. ŌĆó Remedies: ’āśEnsure proper amount and uniformity of binder. ’āśDuring compression, maintain room temperature and RH. 17
- 18. Copyright ┬® Keva Pharmaceuticals Friability ’ü▒ Friability is the tendency of a tablet to crumble or break when subjected to mechanical stress. The causes of high friability include inadequate binder, Improper drying of granules, and tablet hardness. ’ü▒ The remedies for high friability include: control of binder, moisture and compression pressure. 18
- 19. Copyright ┬® Keva Pharmaceuticals Conclusion ’üČThese visual defects can reduce the acceptability by the users and patient compliance of the product. In this slide, defects, causes and measures to overcome these defects have been discussed. ’üČThe focus of this discussion is to establish ways to resolve common defects during the tablet compression, and to identify the root cause of each. 19
- 20. Copyright ┬® Keva Pharmaceuticals REFRENCES 1.Lachman L., Liberman, H.A., Joseph L. K. The Theory and Practice of Industrial Pharmacy; Varghese Publishing House; Mumbai; Third Edition 297-321. 3.Aulton M. pharmaceutics: The science of Dosage Form Design; International Student Edition: 304-321, 347-668. 4.Remington J. Remington: The Science and Practice of Pharmacy; Nineteenth Edition:Volume ŌģĪ 1615-1641. 5. Ansel H., Allen L., Jr. Popovich N. AnselŌĆÖs Pharmaceutical Dosage Forms and Drug Delivery System; Eighth Edition:227-259. 6. Mohrle R ŌĆ£Effervescent tabletsŌĆØ in Liberman, Lachman, Lachman L. Schwartz. Pharmaceutical dosage form, ŌĆ£TabletsŌĆØ vol-ŌģĀ first Indian reprint (2005) Marcel dekkar inc New York -285-292. 20
- 21. Copyright ┬® Keva Pharmaceuticals 21
- 22. Copyright ┬® Keva Pharmaceuticals 22