Types of polymerase chain reactions (APEH Daniel O.)
- 1. TYPES OF POLYMERASE CHAIN REACTIONS Apeh Daniel O. 14TH DEC. 2012
- 2. INTRODUCTION ’āśPCR is an in vitro technique that uses a few basic everyday molecular biology reagents to make large numbers of copies of a specific DNA fragment or a specific region of a DNA strand in a test-tube. ’āśDNA amplification has several applications ’āśThe types are numerous and unclassified ’ü▒Possible Bases for Classification ’é¦Specificity/Error elimination ’é¦Where they take place ’é¦Application areas ’é¦Target ’é¦Similarity in methodology ’é¦Environmental factors e.t.c
- 3. REQUIREMENTS FOR PCR ’āśDNA Template ’āśPrimers ’āśTaq polymerase ’āśDeoxynucleoside ’āśtriphosphates(dNTPs) ’āśBuffer solution ’āśDivalent cations(eg.Mg2+ ) 3
- 4. NEW AUTOMATED PCR OLD PCR 4
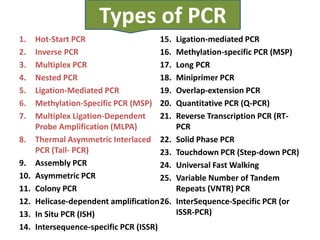
- 5. Types of PCR 1. Hot-Start PCR 15. Ligation-mediated PCR 2. Inverse PCR 16. Methylation-specific PCR (MSP) 3. Multiplex PCR 17. Long PCR 4. Nested PCR 18. Miniprimer PCR 5. Ligation-Mediated PCR 19. Overlap-extension PCR 6. Methylation-Specific PCR (MSP) 20. Quantitative PCR (Q-PCR) 7. Multiplex Ligation-Dependent 21. Reverse Transcription PCR (RT- Probe Amplification (MLPA) PCR 8. Thermal Asymmetric Interlaced 22. Solid Phase PCR PCR (Tail- PCR) 23. Touchdown PCR (Step-down PCR) 9. Assembly PCR 24. Universal Fast Walking 10. Asymmetric PCR 25. Variable Number of Tandem 11. Colony PCR Repeats (VNTR) PCR 12. Helicase-dependent amplification 26. InterSequence-Specific PCR (or 13. In Situ PCR (ISH) ISSR-PCR) 14. Intersequence-specific PCR (ISSR)
- 6. Inverse PCR ’āś In this method amplification of DNA of unknown sequence is carried out from known sequence. ’āś This is especially useful in amplifying and identifying flanking sequences of various genomic inserts. Source: Randy et al., 2007
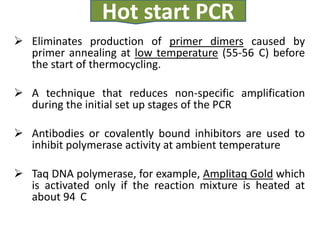
- 7. Hot start PCR ’āś Eliminates production of primer dimers caused by primer annealing at low temperature (55-56 C) before the start of thermocycling. ’āś A technique that reduces non-specific amplification during the initial set up stages of the PCR ’āś Antibodies or covalently bound inhibitors are used to inhibit polymerase activity at ambient temperature ’āś Taq DNA polymerase, for example, Amplitaq Gold which is activated only if the reaction mixture is heated at about 94 C
- 8. Source: Sujatha R (2011) et al 2011
- 9. Multiplex PCR ’āśTechnique for amplification of multiple targets in a single PCR experiment ’āśUses multiple pairs of primers to amplify many sequences simultaneously. ’āśPrimers are designed to have similar annealing temperatures. ’āśSaves time and effort ’āśApplied in Mutation Analysis, Gene Deletion Analysis
- 10. Source: Engelstad et al., 2003
- 11. Nested PCR ’āś This PCR increases the specificity of DNA amplification ’āś Two sets (instead of one pair) of primers are used in two successive PCRs ’āś In the first reaction, one pair of primers ŌĆ£outer pairŌĆØ is used to generate DNA products ’āś The product(s) are then used in a second PCR after the reaction is diluted with a set of second set ŌĆ£nested or internalŌĆØ primers whose binding sites are completely or partially different. ’āś The specificity of PCR is determined by the specificity of the PCR primers
- 12. Source: Fraga MF and Esteller M (2002)
- 13. Ligation-Mediated PCR ŌĆó This method uses small DNA oligonucleotide 'linkers' (or adaptors) that are first ligated to fragments of the target DNA. ŌĆó PCR primers that anneal to the linker sequences are then used to amplify the target fragments. ŌĆó This method is deployed for DNA sequencing, genome walking, and DNA footprinting A related technique is Amplified fragment length polymorphism, which generates diagnostic fragments of a genome
- 14. Source: Engelstad et al., 2003
- 15. OTHER PCR TYPES WORTHY OF NOTE ŌĆó Helicase-dependent amplification: similar to traditional PCR, but uses a constant temperature rather than cycling through denaturation and annealing/extension cycles. DNA helicase, an enzyme that unwinds DNA, is used in place of thermal denaturation. ŌĆó Colony PCR the screening of bacterial (E.Coli) or yeast clones for correct ligation or plasmid products. Selected colonies of bacteria or yeast are picked with a sterile toothpick or pipette tip from a growth (agarose) plate. This is then inserted into the PCR master mix or pre- inserted into autoclaved water. PCR is then conducted to determine if the colony contains the DNA fragment or plasmid of interest.
- 16. Conclusion ŌĆó Numerous types of PCR exist and the basis on which they exist vary; ranging from their application areas, specificity, amplification target e.t.c. These all combined, gives us an array of impossibilities in exploitation of genes in various fields of life.