Unit 1_crystal structures.pptx
- 1. crystalline and non-crystalline materials • Solids exist in nature in two principal forms: crystalline and non-crystalline (amorphous) Crystalline material: • Ordered arrangement (long range periodicity) of their ions, atoms or molecules • Repeating periodic array over large atomic distances • Crystals exhibit sharp melting point • Any single crystal- a single grain – no grain boundaries • Most crystalline solids – more grains – polycrystalline • Whiskers – single crystals - dia/thickness to length ratio- very high Crystalline Single crystal Whiskers
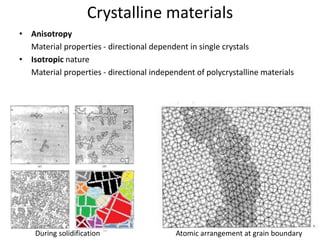
- 2. Crystalline materials • Anisotropy Material properties - directional dependent in single crystals • Isotropic nature Material properties - directional independent of polycrystalline materials During solidification Atomic arrangement at grain boundary
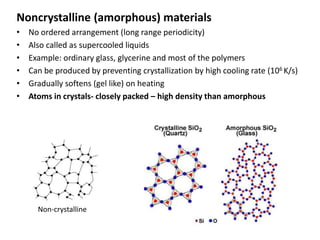
- 3. Noncrystalline (amorphous) materials • No ordered arrangement (long range periodicity) • Also called as supercooled liquids • Example: ordinary glass, glycerine and most of the polymers • Can be produced by preventing crystallization by high cooling rate (106 K/s) • Gradually softens (gel like) on heating • Atoms in crystals- closely packed – high density than amorphous Non-crystalline
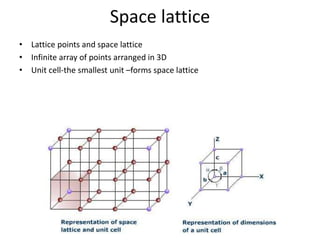
- 4. Space lattice • Lattice points and space lattice • Infinite array of points arranged in 3D • Unit cell-the smallest unit –forms space lattice
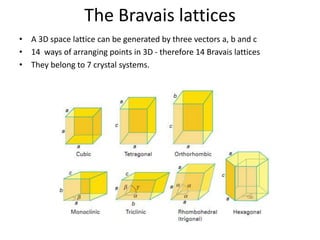
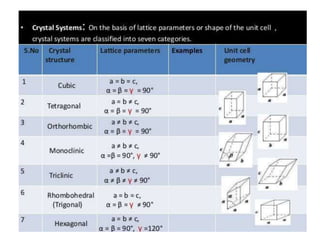
- 5. The Bravais lattices • A 3D space lattice can be generated by three vectors a, b and c • 14 ways of arranging points in 3D - therefore 14 Bravais lattices • They belong to 7 crystal systems.
- 7. 14 ways of arranging points in 3D - therefore 14 Bravais lattices
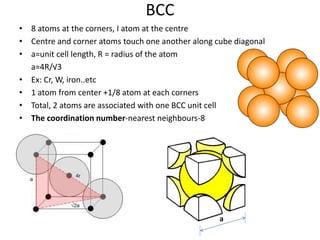
- 8. BCC • 8 atoms at the corners, I atom at the centre • Centre and corner atoms touch one another along cube diagonal • a=unit cell length, R = radius of the atom a=4R/√3 • Ex: Cr, W, iron..etc • 1 atom from center +1/8 atom at each corners • Total, 2 atoms are associated with one BCC unit cell • The coordination number-nearest neighbours-8 a a
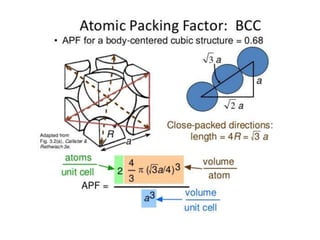
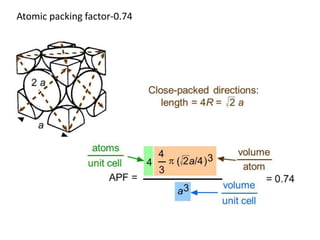
- 9. Atomic packing factor (APF): = Volume of atoms in a unit cell/total unit cell volume = Vs/Vc BCC unit cell - APF=0.68 Vs = average number of atoms (n) X volume of one atom (Va) Simple Cubic structure – APC= 0.52 Va = (4/3) * π r3 APF = (nVa)/Vc
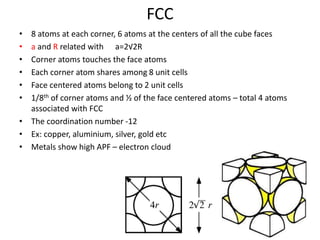
- 12. FCC • 8 atoms at each corner, 6 atoms at the centers of all the cube faces • a and R related with a=2√2R • Corner atoms touches the face atoms • Each corner atom shares among 8 unit cells • Face centered atoms belong to 2 unit cells • 1/8th of corner atoms and ½ of the face centered atoms – total 4 atoms associated with FCC • The coordination number -12 • Ex: copper, aluminium, silver, gold etc • Metals show high APF – electron cloud
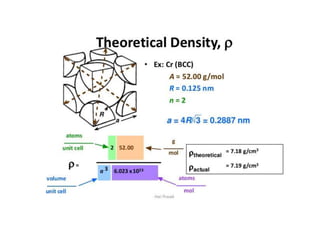
- 14. • We can calculate theoretical density, if the crystal structure is known Density ρ = n A/Vc NA n = number of atoms associated with each unit cell A = atomic weight Vc =Volume of the unit cell NA= Avagadro’s number (6.023 X 1023 atoms/mole)
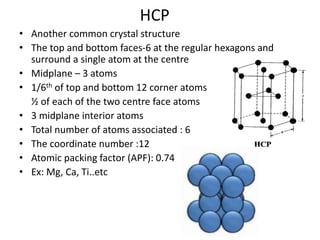
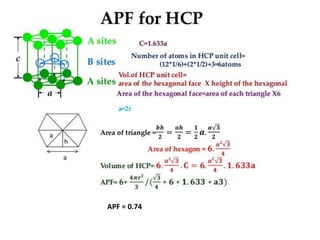
- 16. HCP • Another common crystal structure • The top and bottom faces-6 at the regular hexagons and surround a single atom at the centre • Midplane – 3 atoms • 1/6th of top and bottom 12 corner atoms ½ of each of the two centre face atoms • 3 midplane interior atoms • Total number of atoms associated : 6 • The coordinate number :12 • Atomic packing factor (APF): 0.74 • Ex: Mg, Ca, Ti..etc
- 17. APF = 0.74
- 18. Summary • Stability-metastability • Atomic bonds • Crystalline and non-crystalline materials • Space lattice, unitcell • Different crystal structures • BCC, FCC and hcp structures