Valency table
- 1. mehedi.farazi33@gmail.com VALENCY TABLE Valency One Valency Two Valency Three Valency Four Valency Five Valency Six H O N C N S F S P S P Cl C Al Sn(ic) As(ic) Br Ca Fe(ic) Pb(ic) Sb(ic) I Mg As(ous) U V Na Cu(ic) Sb(ous) Mn K Fe(ous) Cr Si Cu (ous) Zn Au(ic) Ag Sn(ous) B Au(ous) Pb(ous) Bi Hg(ous) Cd Co Ba Hg(ic) Mn (: RA-------- ----DI-- -----CL---- ---ES-----): NH4 + CO3 2- PO3 3- [Fe(CN)6]4- OH- SO3 2- PO4 3- NO2 - SO4 2- [Fe(CN)6]3- NO3 - CrO4 2- BO3 3- HCO3 - Cr2O7 2- Al(OH)4 - SiO3 2- ClO3 - Zn(OH)4 2- CNS- S2O3 2- ClO4 - MnO4 - PH4 + HSO3 - HSO4 - CN- CN0- OCl-
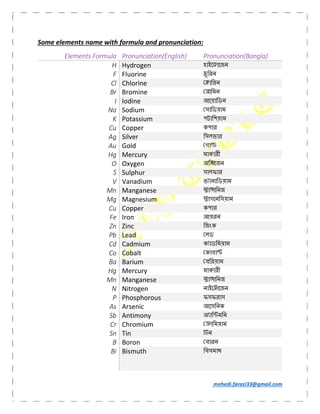
- 2. mehedi.farazi33@gmail.com Some elements name with formula and pronunciation: Elements Formula Pronunciation(English) Pronunciation(Bangla) H Hydrogen হাইে ােজন F Fluorine ুিরন Cl Chlorine ািরন Br Bromine ািমন I Iodine আেয়ািডন Na Sodium সািডয়াম K Potassium পটািশয়াম Cu Copper কপার Ag Silver িসলভার Au Gold গা Hg Mercury মাকারী O Oxygen অি েজন S Sulphur সালফার V Vanadium ভ ানািডয়াম Mn Manganese া ািনজ Mg Magnesium াগেনিসয়াম Cu Copper কপার Fe Iron আয়রন Zn Zinc িজংক Pb Lead লড Cd Cadmium ক াডিময়াম Co Cobalt কাবা Ba Barium বিরয়াম Hg Mercury মাকারী Mn Manganese া ািনজ N Nitrogen নাইে ােজন P Phosphorous ফসফরাস As Arsenic আেসিনক Sb Antimony অ াি মিন Cr Chromium ািময়াম Sn Tin ন B Boron বারন Bi Bismuth িবসমাথ
- 3. mehedi.farazi33@gmail.com Radicals(েযৗগমূলক) Name (In English) Name(In Bangla) NH4 + Ammonium ion অ ােমািনয়াম OH- Hydroxyl হাইে াি ল NO2 - Nitrite নাই াইট NO3 - Nitrate নাইে ট HCO3 - Bicarbonate বাইকাবেনট Al(OH)4 - Aluminate অ ালুিমেনট ClO3 - Chlorate ােরট CNS- Thyo-cyanate থােয়াসায়ােনট ClO4 - Per-Chlorate পারে ােরট MnO4 - Per-Manganate পার া ােনট PH4 + Phosphonium ফসেফািনয়াম HSO3 - Bi-Sulphite বাই-সালফাইট HSO4 - Bi-Sulphate বাই-সালেফট CN- Cyanide সায়ানাইড CN0- Cyanate সায়ােনট OCl- Hypo-Chloride হাইেপাে ারাইড CO3 2- Carbonate কাবেনট SO3 2- Sulphite সালফাইট SO3 2- Sulphate সালেফট CrO4 2- Chromate ােমট Cr2O7 2- Di-Chromate ডাই-ে ােমট SiO3 2- Silicate িসিলেকট Zn(OH)4 2- Zincate িজে ট S2O3 2- Thyo-Sulphate থােয়া-সালেফট PO3 3- Phosphite ফসফাইট PO4 3- Phosphate ফসেফট [Fe(CN)6]3- Ferri-Cyanide ফির-সায়ানাইড BO3 3- Borate বােরট [Fe(CN)6]4- Ferro-Cyanide ফেরা-সায়ানাইড

![mehedi.farazi33@gmail.com
VALENCY TABLE
Valency One Valency Two Valency
Three
Valency Four Valency Five Valency Six
H O N C N S
F S P S P
Cl C Al Sn(ic) As(ic)
Br Ca Fe(ic) Pb(ic) Sb(ic)
I Mg As(ous) U V
Na Cu(ic) Sb(ous) Mn
K Fe(ous) Cr Si
Cu (ous) Zn Au(ic)
Ag Sn(ous) B
Au(ous) Pb(ous) Bi
Hg(ous) Cd
Co
Ba
Hg(ic)
Mn
(: RA-------- ----DI-- -----CL---- ---ES-----):
NH4
+ CO3
2- PO3
3- [Fe(CN)6]4-
OH- SO3
2- PO4
3-
NO2
- SO4
2- [Fe(CN)6]3-
NO3
- CrO4
2- BO3
3-
HCO3
- Cr2O7
2-
Al(OH)4
- SiO3
2-
ClO3
- Zn(OH)4
2-
CNS- S2O3
2-
ClO4
-
MnO4
-
PH4
+
HSO3
-
HSO4
-
CN-
CN0-
OCl-](https://image.slidesharecdn.com/valencytable-160315174208/85/Valency-table-1-320.jpg)
![mehedi.farazi33@gmail.com
Radicals(েযৗগমূলক) Name (In English) Name(In Bangla)
NH4
+
Ammonium ion অ ােমািনয়াম
OH-
Hydroxyl হাইে াি ল
NO2
-
Nitrite নাই াইট
NO3
-
Nitrate নাইে ট
HCO3
-
Bicarbonate বাইকাবেনট
Al(OH)4
-
Aluminate অ ালুিমেনট
ClO3
-
Chlorate ােরট
CNS-
Thyo-cyanate থােয়াসায়ােনট
ClO4
-
Per-Chlorate পারে ােরট
MnO4
-
Per-Manganate পার া ােনট
PH4
+
Phosphonium ফসেফািনয়াম
HSO3
-
Bi-Sulphite বাই-সালফাইট
HSO4
-
Bi-Sulphate বাই-সালেফট
CN-
Cyanide সায়ানাইড
CN0-
Cyanate সায়ােনট
OCl-
Hypo-Chloride হাইেপাে ারাইড
CO3
2-
Carbonate কাবেনট
SO3
2-
Sulphite সালফাইট
SO3
2-
Sulphate সালেফট
CrO4
2-
Chromate ােমট
Cr2O7
2-
Di-Chromate ডাই-ে ােমট
SiO3
2-
Silicate িসিলেকট
Zn(OH)4
2-
Zincate িজে ট
S2O3
2-
Thyo-Sulphate থােয়া-সালেফট
PO3
3-
Phosphite ফসফাইট
PO4
3-
Phosphate ফসেফট
[Fe(CN)6]3-
Ferri-Cyanide ফির-সায়ানাইড
BO3
3-
Borate বােরট
[Fe(CN)6]4-
Ferro-Cyanide ফেরা-সায়ানাইড](https://image.slidesharecdn.com/valencytable-160315174208/85/Valency-table-3-320.jpg)