writing ionic compounds in chemistry.pptx
Download as PPTX, PDF0 likes12 views
writing ionic compounds
1 of 14
Download to read offline






Ad
Recommended
Ionic nomenclature
Ionic nomenclatureCurrituck County High School
Ã˝
This document provides information on naming ionic compounds. It discusses that ionic compounds are made of positive and negative ions. It explains how to name binary ionic compounds containing two elements and ternary compounds containing three elements, with two elements in a polyatomic ion. It provides examples of naming binary ionic compounds like NaCl and ternary compounds like Mg(OH)2. It also discusses writing formulas from compound names.BINARY IONIC COMPOUND.pptx
BINARY IONIC COMPOUND.pptxRomuloJrBarrameda
Ã˝
This document provides information on binary compounds and ionic compounds containing polyatomic ions. It defines binary compounds as those containing only two elements and ionic binary compounds as those where one element is a metal and the other is a non-metal. Rules are provided for naming ionic compounds containing metals and non-metals or polyatomic ions. Key points include that polyatomic ions are charged groups that don't occur alone and ionic compounds contain positive and negative ions to be neutral overall. Formulas show how to write names and formulas for ionic compounds containing polyatomic ions.Vi nomenclature of inorganic compounds
Vi nomenclature of inorganic compoundsOmar Betanzos
Ã˝
The document discusses the nomenclature of inorganic compounds according to IUPAC rules. It covers naming conventions for binary ionic compounds such as metal oxides, salts, metal hydrides, non-metal halides, and binary acids. It also discusses determining oxidation states and writing chemical formulas. Key points include naming metal oxides as "stock name of metal cation" + "oxide", salts as "stock name of metal cation" + "root name of non-metal anion" + "-ide", and distinguishing between ionic and molecular compounds in nomenclature.2012 ppt unit 2 3 ionic bonding djy r1
2012 ppt unit 2 3 ionic bonding djy r1David Young
Ã˝
Ionic bonding results from the electrostatic attraction between oppositely charged ions. When a metal atom reacts with a nonmetal, the metal typically loses electrons to form a cation while the nonmetal gains electrons to form an anion. These oppositely charged ions are then attracted to each other, forming an ionic bond. Ionic compounds have crystalline structures where the ions are arranged in repeating patterns. Their strong ionic bonds make them brittle with high melting points. Many ionic compounds dissolve in water to form electrolyte solutions where the ions are free to move.NWTC General Chemistry Ch 06
NWTC General Chemistry Ch 06Steve Sinclair
Ã˝
This chapter discusses the nomenclature of inorganic compounds. It covers common and systematic naming of compounds, writing formulas from names of ionic compounds, and naming binary ionic compounds. The key points are:
- Common names are arbitrary while systematic names precisely identify chemical composition.
- Ions are named by changing the element name or using stock/classic prefixes depending on the charge. Formulas are written by balancing charges between cations and anions.
- Binary ionic compounds are named by identifying the cation and anion, including charge information for transition metals."Chemical Bonding - for medical students
"Chemical Bonding - for medical studentsJemaimaNgayyacGuday
Ã˝
The document discusses various types of chemical bonds including ionic, covalent, and metallic bonds, as well as intermolecular forces such as hydrogen bonding and dipole-dipole interactions. It outlines the formation and properties of these bonds, and provides nomenclature rules for naming ionic and molecular compounds, including the use of prefixes and Roman numerals for transition metals. Additionally, it explains how to identify and name binary acids based on their chemical formulas.Chapter 9
Chapter 9SHERIFA s
Ã˝
This document provides information on naming and writing formulas for different types of chemical compounds including:
1. Ionic compounds consisting of monatomic and polyatomic ions are named by writing the cation first followed by the anion. Transition metal ions have stock and classical naming systems.
2. Molecular compounds consisting of two nonmetals are named using prefixes to indicate the number of atoms of each element followed by the "-ide" suffix.
3. Acids are named based on the anion present, using prefixes like "hydro-", "-ous", or "-ic" depending on the anion suffix. Their formulas are written with hydrogen and the anion.
4. Bases are named by writing the cationChemical Bonding - Holding Particles Together.pptx
Chemical Bonding - Holding Particles Together.pptxRhodaF
Ã˝
The document covers chemical bonding, including the nature and properties of substances influenced by atomic structure and bonds. It discusses various types of chemical bonds such as ionic, covalent, and metallic, along with the octet rule, valence electrons, and how to determine chemical formulas and names for compounds. Additionally, it reviews key concepts such as electronegativity, ionization energy, molecular geometry, and the distinctions between ionic and covalent bonding.2011 2012 Chapter 5 Review
2011 2012 Chapter 5 Reviewmegonigale
Ã˝
The document discusses ionic and metallic bonding. Ions are formed when atoms gain or lose electrons to achieve a noble gas configuration. Ionic bonds form when cations (positively charged ions) and anions (negatively charged ions) are attracted via electrostatic forces. Metallic bonds occur in metals and involve delocalized electrons that provide conductivity and allow metals to be malleable. Ionic compounds are named by writing the cation name followed by the anion name.NOMENCLATURE OF BINARY POLY-ATOMIC COMPOUNDS
NOMENCLATURE OF BINARY POLY-ATOMIC COMPOUNDSGrinty Babu
Ã˝
The document outlines the nomenclature and rules for naming chemical compounds, specifically ionic, binary molecular, and ternary ionic compounds. It explains how to determine charges of ions, use of Roman numerals for transition metals, and how to balance chemical formulas. Additionally, the document provides tips for identifying and naming various types of compounds, including the use of prefixes for binary molecular compounds.PP_15a_chp_2_8_naming_ionic_compounds.ppt
PP_15a_chp_2_8_naming_ionic_compounds.pptDavidJenil
Ã˝
The document discusses naming simple ionic compounds. It provides rules for naming type I and type II binary ionic compounds. For type I compounds, the cation is named first followed by the anion with the "-ide" suffix. For type II compounds containing metals with multiple charges, the roman numeral for the metal's charge is included in parentheses. The document also discusses naming compounds containing polyatomic ions by following similar rules.Naming Ionic and Covalent Compounds
Naming Ionic and Covalent CompoundsJohn Canuel
Ã˝
1. The document provides an overview of writing formulas and naming ionic and covalent compounds. It reviews the periodic table and properties of metals, nonmetals and metalloids.
2. Key concepts covered include ion formation, the octet rule, polyatomic ions, oxidation numbers, naming conventions for ionic compounds containing metals or transition metals, and prefixes used in naming covalent compounds.
3. The document distinguishes between ionic and covalent bonding, lattice structures, and molecular structures of compounds.Ionic Bonds - Chapter 7
Ionic Bonds - Chapter 7Galen West
Ã˝
Ionic bonding occurs when atoms transfer electrons to form ions with opposite charges that are attracted via electrostatic forces. Metals form cations by losing electrons to achieve stable electron configurations like noble gases, while nonmetals form anions by gaining electrons. This transfer of electrons allows the formation of ionic compounds with crystalline structures where ion attractions are maximized and repulsions minimized. Properties of ionic compounds include high melting points, solubility in water, defined crystal structures, and the ability to conduct electricity when molten. Metallic bonding also involves cations but is characterized by delocalized valence electrons that form a "sea" allowing metals to conduct electricity and be malleable and ductile.Lecture 9.1 & 9.2- Naming Salts
Lecture 9.1 & 9.2- Naming SaltsMary Beth Smith
Ã˝
The document discusses chemical names and formulas of ions. It explains that monatomic cations form when metals lose electrons and gain a positive charge equal to their group number. Monatomic anions form when non-metals gain electrons and have a negative charge equal to the group number minus 8. Polyatomic ions consist of multiple atoms and have an overall positive or negative charge. Transition metals can form multiple ions with different charges that must be specified. Ionic compounds are named by writing the cation first followed by the anion.Grade 9 chemistry, ions and writing chemical formulae
Grade 9 chemistry, ions and writing chemical formulaeNellexo
Ã˝
This document provides information on writing chemical formulas for ionic compounds. It discusses how ions are formed by elements gaining or losing electrons to achieve stability like the nearest noble gas. The charge on simple ions relates to the number of electrons gained or lost. Polyatomic ions contain two or more combined atoms and usually have a negative charge except for ammonium. To write formulas for ionic compounds, the numbers of positive and negative ions must balance to give an electrically neutral compound. The names of ionic compounds consist of the cation name followed by the anion name changed to end in "-ide".Ch 8 ionic compounds
Ch 8 ionic compoundsEsther Herrera
Ã˝
This document discusses ionic compounds and the formation of ionic bonds. Ions form when atoms gain or lose electrons to achieve stable electron configurations like noble gases. Ionic bonds occur between oppositely charged ions and result in crystalline solids with high melting points. The document explains how to name ionic compounds based on the cation and anion present and write chemical formulas from compound names. It also briefly discusses metallic bonding and the properties of metals and alloys.Ionic Naming
Ionic NamingGary Abud Jr
Ã˝
The document provides information on naming ionic compounds, cations, anions, and polyatomic ions. It discusses how to name monatomic and transition metal cations by adding "-ion" or the charge in Roman numerals after the element name. Anions are named by adding "-ide" after the element root. Ionic compounds are formed by writing the cation first followed by the anion. Polyatomic ions are also included.Naming_and_Writing_in_Applied_Chemistry.
Naming_and_Writing_in_Applied_Chemistry.KaitlynneMaeGamboa
Ã˝
This document covers the naming and writing of chemical formulas for ionic and molecular compounds, including the rules for constructing their names based on the elements involved. It explains how to identify cations and anions, the use of prefixes in molecular compounds, and how to handle transition metals with multiple charges. Additionally, it includes assessment questions to reinforce understanding of the material.Ions and ionic bonding
Ions and ionic bondingK Lipinski
Ã˝
1. The document describes a scientist working to transport a zombie virus cure from their lab to the CDC for mass distribution.
2. With zombies already walking the earth, the scientist must devise a plan to get the cure to where it's needed before it's too late.
3. The scientist is asked to write a journal entry describing their plan or encounter with zombies during the transport of the cure.Chapter 7.1 : Chemical Names and Formulas
Chapter 7.1 : Chemical Names and FormulasChris Foltz
Ã˝
Chemical formulas indicate the relative number and type of atoms in a chemical compound or molecule. A chemical formula for an ionic compound represents one formula unit and uses the simplest whole number ratio of ions present. Binary ionic compounds are composed of two elements and the total positive charges must equal the total negative charges. Molecular compounds use a prefix system to indicate the number of atoms of the less electronegative element present. Polyatomic ions are named as units within chemical formulas.Ch 8 ionic compounds
Ch 8 ionic compoundsEsther Herrera
Ã˝
Ionic compounds form when a metal transfers electrons to a nonmetal, resulting in oppositely charged ions that are attracted in a crystal lattice structure. The ratio of cations to anions must result in an overall neutral charge. Ionic compounds are named by writing the cation first followed by the anion. Polyatomic ions are named based on the nonmetal and number of oxygen atoms. Metallic bonds form a "sea of electrons" that are delocalized and allow metal atoms to strongly attract each other.G9 Science Q2-Week 2- Types of Compounds.ppt
G9 Science Q2-Week 2- Types of Compounds.pptElliePamaPastrana
Ã˝
The document details the comparison of ionic, covalent, and metallic compounds, explaining their bonding characteristics, properties, and the behavior of ions. It discusses how atoms combine through ionic and covalent bonds, the formation of cations and anions, and the energy changes associated with bond formation and breaking. Additionally, it covers the classification and naming of these compounds while providing insights into the physical properties of ionic and molecular substances.Ionic Compounds
Ionic CompoundsOhMiss
Ã˝
The document discusses ionic compounds and their properties. It defines ionic compounds as formed from positively and negatively charged ions. Sodium chloride is given as an example where sodium loses an electron to become Na+ and chlorine gains an electron to become Cl-. Ionic compounds form crystalline structures and dissolve into ions in water, allowing them to conduct electricity. Common polyatomic ions are also discussed.GENCHEM1-nomenclature.pptx
GENCHEM1-nomenclature.pptxNurulhayahMarhamahMa
Ã˝
Nomenclature is the process of naming chemical compounds with systematic names so they can be easily identified. This document discusses the naming of ionic compounds, including binary ionic compounds composed of a metal and nonmetal, and ternary ionic compounds containing a polyatomic ion. It explains that the cation is always named first and the anion name ends in "-ide" for binary ionic compounds. For transition metals, Roman numerals indicate the ion charge. Ternary compounds contain two ions, with at least one being a polyatomic ion, and both ions are simply named in order.Chemistry - Chp 9 - Chemical Names and Formulas - PowerPoint
Chemistry - Chp 9 - Chemical Names and Formulas - PowerPointMr. Walajtys
Ã˝
This document covers naming and writing formulas for ions, ionic compounds, molecular compounds, acids, and bases. It provides objectives and definitions for each section, examples of naming and writing formulas, and explanations of naming conventions and rules including prefixes, charges, and endings for different types of compounds.Applied Chapter 5.3 : Names and Formulas of Compounds
Applied Chapter 5.3 : Names and Formulas of CompoundsChris Foltz
Ã˝
This document discusses naming and writing formulas for ionic compounds and compounds containing polyatomic ions. It explains that ionic compounds are made up of positive and negative ions in the simplest ratio to achieve neutral charge. Formulas show the types and numbers of ions present. Names specify the cation first followed by the anion. Polyatomic ions are named and treated as a single unit. Examples are provided to illustrate naming and writing formulas for various ionic compounds and those containing common polyatomic ions.Chemical Names and Formulas
Chemical Names and FormulasCurrituck County High School
Ã˝
This document discusses classifying and naming ionic and covalent compounds, as well as writing their formulas. It provides rules for:
- Classifying compounds as ionic or covalent based on their formula
- Naming ionic compounds using stock systems and identifying polyatomic ions
- Naming covalent compounds using prefixes to indicate the number of atoms
- Writing formulas for ionic compounds by balancing charges and for covalent compounds using prefixes
It also discusses acids, bases, and how to name and write formulas for acids based on their anion name endings.Fomulas of Common Chemical Substances.pptx
Fomulas of Common Chemical Substances.pptxMaryAnnLazarteBesar
Ã˝
The document outlines the fundamentals of writing and naming chemical formulas, focusing on cations, anions, ionic and covalent compounds, and acids. It includes rules for identifying elements, balancing charges, and using prefixes for covalent compounds, as well as methods for naming polyatomic ions. Additionally, it emphasizes the importance of chemical formulas for understanding the composition and reactions of substances.More Related Content
Similar to writing ionic compounds in chemistry.pptx (20)
2011 2012 Chapter 5 Review
2011 2012 Chapter 5 Reviewmegonigale
Ã˝
The document discusses ionic and metallic bonding. Ions are formed when atoms gain or lose electrons to achieve a noble gas configuration. Ionic bonds form when cations (positively charged ions) and anions (negatively charged ions) are attracted via electrostatic forces. Metallic bonds occur in metals and involve delocalized electrons that provide conductivity and allow metals to be malleable. Ionic compounds are named by writing the cation name followed by the anion name.NOMENCLATURE OF BINARY POLY-ATOMIC COMPOUNDS
NOMENCLATURE OF BINARY POLY-ATOMIC COMPOUNDSGrinty Babu
Ã˝
The document outlines the nomenclature and rules for naming chemical compounds, specifically ionic, binary molecular, and ternary ionic compounds. It explains how to determine charges of ions, use of Roman numerals for transition metals, and how to balance chemical formulas. Additionally, the document provides tips for identifying and naming various types of compounds, including the use of prefixes for binary molecular compounds.PP_15a_chp_2_8_naming_ionic_compounds.ppt
PP_15a_chp_2_8_naming_ionic_compounds.pptDavidJenil
Ã˝
The document discusses naming simple ionic compounds. It provides rules for naming type I and type II binary ionic compounds. For type I compounds, the cation is named first followed by the anion with the "-ide" suffix. For type II compounds containing metals with multiple charges, the roman numeral for the metal's charge is included in parentheses. The document also discusses naming compounds containing polyatomic ions by following similar rules.Naming Ionic and Covalent Compounds
Naming Ionic and Covalent CompoundsJohn Canuel
Ã˝
1. The document provides an overview of writing formulas and naming ionic and covalent compounds. It reviews the periodic table and properties of metals, nonmetals and metalloids.
2. Key concepts covered include ion formation, the octet rule, polyatomic ions, oxidation numbers, naming conventions for ionic compounds containing metals or transition metals, and prefixes used in naming covalent compounds.
3. The document distinguishes between ionic and covalent bonding, lattice structures, and molecular structures of compounds.Ionic Bonds - Chapter 7
Ionic Bonds - Chapter 7Galen West
Ã˝
Ionic bonding occurs when atoms transfer electrons to form ions with opposite charges that are attracted via electrostatic forces. Metals form cations by losing electrons to achieve stable electron configurations like noble gases, while nonmetals form anions by gaining electrons. This transfer of electrons allows the formation of ionic compounds with crystalline structures where ion attractions are maximized and repulsions minimized. Properties of ionic compounds include high melting points, solubility in water, defined crystal structures, and the ability to conduct electricity when molten. Metallic bonding also involves cations but is characterized by delocalized valence electrons that form a "sea" allowing metals to conduct electricity and be malleable and ductile.Lecture 9.1 & 9.2- Naming Salts
Lecture 9.1 & 9.2- Naming SaltsMary Beth Smith
Ã˝
The document discusses chemical names and formulas of ions. It explains that monatomic cations form when metals lose electrons and gain a positive charge equal to their group number. Monatomic anions form when non-metals gain electrons and have a negative charge equal to the group number minus 8. Polyatomic ions consist of multiple atoms and have an overall positive or negative charge. Transition metals can form multiple ions with different charges that must be specified. Ionic compounds are named by writing the cation first followed by the anion.Grade 9 chemistry, ions and writing chemical formulae
Grade 9 chemistry, ions and writing chemical formulaeNellexo
Ã˝
This document provides information on writing chemical formulas for ionic compounds. It discusses how ions are formed by elements gaining or losing electrons to achieve stability like the nearest noble gas. The charge on simple ions relates to the number of electrons gained or lost. Polyatomic ions contain two or more combined atoms and usually have a negative charge except for ammonium. To write formulas for ionic compounds, the numbers of positive and negative ions must balance to give an electrically neutral compound. The names of ionic compounds consist of the cation name followed by the anion name changed to end in "-ide".Ch 8 ionic compounds
Ch 8 ionic compoundsEsther Herrera
Ã˝
This document discusses ionic compounds and the formation of ionic bonds. Ions form when atoms gain or lose electrons to achieve stable electron configurations like noble gases. Ionic bonds occur between oppositely charged ions and result in crystalline solids with high melting points. The document explains how to name ionic compounds based on the cation and anion present and write chemical formulas from compound names. It also briefly discusses metallic bonding and the properties of metals and alloys.Ionic Naming
Ionic NamingGary Abud Jr
Ã˝
The document provides information on naming ionic compounds, cations, anions, and polyatomic ions. It discusses how to name monatomic and transition metal cations by adding "-ion" or the charge in Roman numerals after the element name. Anions are named by adding "-ide" after the element root. Ionic compounds are formed by writing the cation first followed by the anion. Polyatomic ions are also included.Naming_and_Writing_in_Applied_Chemistry.
Naming_and_Writing_in_Applied_Chemistry.KaitlynneMaeGamboa
Ã˝
This document covers the naming and writing of chemical formulas for ionic and molecular compounds, including the rules for constructing their names based on the elements involved. It explains how to identify cations and anions, the use of prefixes in molecular compounds, and how to handle transition metals with multiple charges. Additionally, it includes assessment questions to reinforce understanding of the material.Ions and ionic bonding
Ions and ionic bondingK Lipinski
Ã˝
1. The document describes a scientist working to transport a zombie virus cure from their lab to the CDC for mass distribution.
2. With zombies already walking the earth, the scientist must devise a plan to get the cure to where it's needed before it's too late.
3. The scientist is asked to write a journal entry describing their plan or encounter with zombies during the transport of the cure.Chapter 7.1 : Chemical Names and Formulas
Chapter 7.1 : Chemical Names and FormulasChris Foltz
Ã˝
Chemical formulas indicate the relative number and type of atoms in a chemical compound or molecule. A chemical formula for an ionic compound represents one formula unit and uses the simplest whole number ratio of ions present. Binary ionic compounds are composed of two elements and the total positive charges must equal the total negative charges. Molecular compounds use a prefix system to indicate the number of atoms of the less electronegative element present. Polyatomic ions are named as units within chemical formulas.Ch 8 ionic compounds
Ch 8 ionic compoundsEsther Herrera
Ã˝
Ionic compounds form when a metal transfers electrons to a nonmetal, resulting in oppositely charged ions that are attracted in a crystal lattice structure. The ratio of cations to anions must result in an overall neutral charge. Ionic compounds are named by writing the cation first followed by the anion. Polyatomic ions are named based on the nonmetal and number of oxygen atoms. Metallic bonds form a "sea of electrons" that are delocalized and allow metal atoms to strongly attract each other.G9 Science Q2-Week 2- Types of Compounds.ppt
G9 Science Q2-Week 2- Types of Compounds.pptElliePamaPastrana
Ã˝
The document details the comparison of ionic, covalent, and metallic compounds, explaining their bonding characteristics, properties, and the behavior of ions. It discusses how atoms combine through ionic and covalent bonds, the formation of cations and anions, and the energy changes associated with bond formation and breaking. Additionally, it covers the classification and naming of these compounds while providing insights into the physical properties of ionic and molecular substances.Ionic Compounds
Ionic CompoundsOhMiss
Ã˝
The document discusses ionic compounds and their properties. It defines ionic compounds as formed from positively and negatively charged ions. Sodium chloride is given as an example where sodium loses an electron to become Na+ and chlorine gains an electron to become Cl-. Ionic compounds form crystalline structures and dissolve into ions in water, allowing them to conduct electricity. Common polyatomic ions are also discussed.GENCHEM1-nomenclature.pptx
GENCHEM1-nomenclature.pptxNurulhayahMarhamahMa
Ã˝
Nomenclature is the process of naming chemical compounds with systematic names so they can be easily identified. This document discusses the naming of ionic compounds, including binary ionic compounds composed of a metal and nonmetal, and ternary ionic compounds containing a polyatomic ion. It explains that the cation is always named first and the anion name ends in "-ide" for binary ionic compounds. For transition metals, Roman numerals indicate the ion charge. Ternary compounds contain two ions, with at least one being a polyatomic ion, and both ions are simply named in order.Chemistry - Chp 9 - Chemical Names and Formulas - PowerPoint
Chemistry - Chp 9 - Chemical Names and Formulas - PowerPointMr. Walajtys
Ã˝
This document covers naming and writing formulas for ions, ionic compounds, molecular compounds, acids, and bases. It provides objectives and definitions for each section, examples of naming and writing formulas, and explanations of naming conventions and rules including prefixes, charges, and endings for different types of compounds.Applied Chapter 5.3 : Names and Formulas of Compounds
Applied Chapter 5.3 : Names and Formulas of CompoundsChris Foltz
Ã˝
This document discusses naming and writing formulas for ionic compounds and compounds containing polyatomic ions. It explains that ionic compounds are made up of positive and negative ions in the simplest ratio to achieve neutral charge. Formulas show the types and numbers of ions present. Names specify the cation first followed by the anion. Polyatomic ions are named and treated as a single unit. Examples are provided to illustrate naming and writing formulas for various ionic compounds and those containing common polyatomic ions.Chemical Names and Formulas
Chemical Names and FormulasCurrituck County High School
Ã˝
This document discusses classifying and naming ionic and covalent compounds, as well as writing their formulas. It provides rules for:
- Classifying compounds as ionic or covalent based on their formula
- Naming ionic compounds using stock systems and identifying polyatomic ions
- Naming covalent compounds using prefixes to indicate the number of atoms
- Writing formulas for ionic compounds by balancing charges and for covalent compounds using prefixes
It also discusses acids, bases, and how to name and write formulas for acids based on their anion name endings.Fomulas of Common Chemical Substances.pptx
Fomulas of Common Chemical Substances.pptxMaryAnnLazarteBesar
Ã˝
The document outlines the fundamentals of writing and naming chemical formulas, focusing on cations, anions, ionic and covalent compounds, and acids. It includes rules for identifying elements, balancing charges, and using prefixes for covalent compounds, as well as methods for naming polyatomic ions. Additionally, it emphasizes the importance of chemical formulas for understanding the composition and reactions of substances.More from RODELAZARES3 (20)
dealing-with-stress as a way of managing .pptx
dealing-with-stress as a way of managing .pptxRODELAZARES3
Ã˝
dealing-with-stress as a way of managingPSAAP-I-A M.power point presentation tx
PSAAP-I-A M.power point presentation txRODELAZARES3
Ã˝
The document emphasizes the significance of acknowledging and expressing feelings to enhance self-awareness and emotional safety in a supportive school environment. It includes breathing exercises and group activities aimed at promoting calmness, relaxation, and self-expression while encouraging respectful listening and sharing among peers. Furthermore, it discusses the benefits of proper breathing and recognizing emotions to improve mental health and interpersonal relationships.INSIDE OUT - PowerPoint Presentation.pptx
INSIDE OUT - PowerPoint Presentation.pptxRODELAZARES3
Ã˝
The document outlines the agenda for a new academic year, encouraging students to embrace the learning journey while acknowledging common emotions like excitement, frustration, and anxiety. It details classroom subjects, grading systems, schedules, and classroom rules to promote a respectful and organized learning environment. The message emphasizes teamwork and support among students to foster a positive atmosphere for overcoming challenges together.seat plan for senior high school .pptx
seat plan for senior high school .pptxRODELAZARES3
Ã˝
The document lists a variety of names, suggesting it may pertain to individuals involved in a specific context or event. There is no additional information or context provided about these individuals. Overall, the document appears to be a simple enumeration of names.dealing-with-stress IN EDUCATIONALS.pptx
dealing-with-stress IN EDUCATIONALS.pptxRODELAZARES3
Ã˝
The document provides a comprehensive overview of mental health, including definitions, causes, symptoms, and treatments for mental disorders, as well as common misconceptions. It emphasizes the importance of mental health in schools for both students and educators, highlighting strategies for promoting well-being and addressing work-related stress. Additionally, it discusses the critical role of parents in supporting their children's mental health and offers practical tips for fostering a positive family environment.Approaches to Manage Stress and Connection Between Stress and Your Lifestyle....
Approaches to Manage Stress and Connection Between Stress and Your Lifestyle....RODELAZARES3
Ã˝
The document discusses various approaches to managing stress, highlighting the distinction between distress and eustress, and emphasizes the role of cognitive appraisal in stress management. It introduces the 4 A's approach: Avoid, Alter, Adapt, and Accept, which offers strategies for handling stressors effectively. It also notes the importance of person-environment fit and the impact of one's emotional responses on stress perception.Pink-and-Green-Playful-Guess-The-Picture-Game-Presentation_20240927_133936_00...
Pink-and-Green-Playful-Guess-The-Picture-Game-Presentation_20240927_133936_00...RODELAZARES3
Ã˝
The document appears to be a list of names, possibly representing a group of individuals or characters. The names include varied combinations that suggest a diverse collection. There is no further context or information provided.INSIDE OUT - PowerPoint Presentation.pptx
INSIDE OUT - PowerPoint Presentation.pptxRODELAZARES3
Ã˝
The document outlines a back-to-school orientation for the 2024-2025 academic year, introducing the teacher, class subjects, and grading system. It highlights the emotional experiences of students, addressing feelings of excitement, frustration, and anxiety while emphasizing support and teamwork. The agenda includes classroom rules, subject introductions, and a structured class schedule.TAGISAN NG TALINO SA RESEARCH IN DAILY.pptx
TAGISAN NG TALINO SA RESEARCH IN DAILY.pptxRODELAZARES3
Ã˝
The document outlines the rules and mechanics for a midterm examination in research for the school year 2023-2024, featuring various types of questions related to research principles, qualitative methods, ethical standards, and citation styles. It covers definitions and characteristics of research methodologies, particularly focusing on qualitative approaches, and includes sample questions for each difficulty level. Additionally, the document includes competitive elements, scoring for teams, and the presentation of answers and results.research-RESEARCH-PROCESS_Chapter-3.pptx
research-RESEARCH-PROCESS_Chapter-3.pptxRODELAZARES3
Ã˝
This document outlines the methodology used in a study. It describes the research design as qualitative and ethnographic. 15 farmers between ages 25-60 who have been farming for at least 10 years were interviewed. Data was also collected through participant observation of farming activities. The study was conducted in Barangay Jumaguicjic, Roxas City, Capiz from January to March 2020. Semi-structured interviews and an evaluation checklist were used as research instruments.dealing- with- mental health stress.pptx
dealing- with- mental health stress.pptxRODELAZARES3
Ã˝
The document provides information on promoting mental health and wellbeing in schools. It discusses the importance of mental health for students' education and development. It also emphasizes the need to support the mental health of teachers and school staff, as they experience high stress levels due to responsibilities and workload. Additionally, the document discusses ways to help students cope with stress, such as through communication, consistency in schedules, and developing self-care plans. It also provides tips for schools to promote the wellbeing of parents and the whole family.Ad
Recently uploaded (20)
How to Manage & Create a New Department in Odoo 18 Employee
How to Manage & Create a New Department in Odoo 18 EmployeeCeline George
Ã˝
In Odoo 18's Employee module, organizing your workforce into departments enhances management and reporting efficiency. Departments are a crucial organizational unit within the Employee module.Wax Moon, Richmond, VA. Terrence McPherson
Wax Moon, Richmond, VA. Terrence McPhersonTerrenceMcPherson1
Ã˝
Wax Moon is an independent record store keeping its foundational foothold in vinyl records by taking in collections and keeping the old 80s aesthetics alive with involvement in its community and participation with record distributors.LDMMIA GRAD Student Check-in Orientation Sampler
LDMMIA GRAD Student Check-in Orientation SamplerLDM & Mia eStudios
Ã˝
Completed Tuesday June 10th.
An Orientation Sampler of 8 pages.
It helps to understand the text behind anything. This improves our performance and confidence.
Your training will be mixed media. Includes Rehab Intro and Meditation vods, all sold separately.
Editing our Vods & New Shop.
Retail under $30 per item. Store Fees will apply. Digital Should be low cost.
I am still editing the package. I wont be done until probably July? However; Orientation and Lecture 1 (Videos) will be available soon. Media will vary between PDF and Instruction Videos.
Thank you for attending our free workshops. Those can be used with any Reiki Yoga training package. Traditional Reiki does host rules and ethics. Its silent and within the JP Culture/Area/Training/Word of Mouth. It allows remote healing but there’s limits for practitioners and masters. We are not allowed to share certain secrets/tools. Some content is designed only for “Masters”. Some yoga are similar like the Kriya Yoga-Church (Vowed Lessons). We will review both Reiki and Yoga (Master symbols) later on. Sounds Simple but these things host Energy Power/Protection.
Imagine This package will be a supplement or upgrade for professional Reiki. You can create any style you need.
‚ô•‚ô•‚ô•
•* ́ ̈ ̧.•
(Job) Tech for students: In short, high speed is essential. (Space, External Drives, virtual clouds)
Fast devices and desktops are important. Please upgrade your technology and office as needed and timely. - MIA J. Tech Dept (Timeless)
‚ô•‚ô•‚ô•
•* ́ ̈ ̧.•
Copyright Disclaimer 2007-2025+: These lessons are not to be copied or revised without the
Author’s permission. These Lessons are designed Rev. Moore to instruct and guide students on the path to holistic health and wellness.
It’s about expanding your Nature Talents, gifts, even Favorite Hobbies.
‚ô•‚ô•‚ô•
•* ́ ̈ ̧.•
First, Society is still stuck in the matrix. Many of the spiritual collective, say the matrix crashed. Its now collapsing. This means anything lower, darker realms, astral, and matrix are below 5D. 5D is thee trend. It’s our New Dimensional plane. However; this plane takes work ethic,
integration, and self discovery. ‚ô•‚ô•‚ô•
•* ́ ̈ ̧.•
We don’t need to slave, mule, or work double shifts to fuse Reiki lol. It should blend naturally within our lifestyles. Same with Yoga. There’s no
need to use all the poses/asanas. For under a decade, my fav exercises are not asanas but Pilates. It’s all about Yoga-meditation when using Reiki. (Breaking old myths.)
Thank You for reading our Orientation Sampler. The Workshop is 14 pages on introduction. These are a joy and effortless to produce/make.Unit- 4 Biostatistics & Research Methodology.pdf
Unit- 4 Biostatistics & Research Methodology.pdfKRUTIKA CHANNE
Ã˝
Blocking and confounding (when a third variable, or confounder, influences both the exposure and the outcome) system for Two-level factorials (a type of experimental design where each factor (independent variable) is investigated at only two levels, typically denoted as "high" and "low" or "+1" and "-1")
Regression modeling (statistical model that estimates the relationship between one dependent variable and one or more independent variables using a line): Hypothesis testing in Simple and Multiple regression models
Introduction to Practical components of Industrial and Clinical Trials Problems: Statistical Analysis Using Excel, SPSS, MINITAB®️, DESIGN OF EXPERIMENTS, R - Online Statistical Software to Industrial and Clinical trial approachEnergy Balances Of Oecd Countries 2011 Iea Statistics 1st Edition Oecd
Energy Balances Of Oecd Countries 2011 Iea Statistics 1st Edition Oecdrazelitouali
Ã˝
Energy Balances Of Oecd Countries 2011 Iea Statistics 1st Edition Oecd
Energy Balances Of Oecd Countries 2011 Iea Statistics 1st Edition Oecd
Energy Balances Of Oecd Countries 2011 Iea Statistics 1st Edition OecdOverview of Off Boarding in Odoo 18 Employees
Overview of Off Boarding in Odoo 18 EmployeesCeline George
Ã˝
When an employee leaves the company, Odoo provides a streamlined offboarding process to ensure all necessary steps are taken. How to Manage Inventory Movement in Odoo 18 POS
How to Manage Inventory Movement in Odoo 18 POSCeline George
Ã˝
Inventory management in the Odoo 18 Point of Sale system is tightly integrated with the inventory module, offering a solution to businesses to manage sales and stock in one united system.Measuring, learning and applying multiplication facts.
Measuring, learning and applying multiplication facts.cgilmore6
Ã˝
∫›∫›fl£s from a presentation by Professor Camilla Gilmore to the Association of Teachers of Mathematics and Mathematics Association Primary Interest group in June 2025.
This gave an overview of two studies that investigated children's multiplication fact knowledge. These studies were part of the SUM research project based at the University of Nottingham and Loughborough University. For more information see www.sumproject.org.ukExploring Ocean Floor Features for Middle School
Exploring Ocean Floor Features for Middle SchoolMarie
Ã˝
This 16 slide science reader is all about ocean floor features. It was made to use with middle school students.
You can download the PDF at thehomeschooldaily.com
Thanks! Marie 2025 June Year 9 Presentation: Subject selection.pptx
2025 June Year 9 Presentation: Subject selection.pptxmansk2
Ã˝
2025 June Year 9 Presentation: Subject selectionPaper 108 | Thoreau’s Influence on Gandhi: The Evolution of Civil Disobedience
Paper 108 | Thoreau’s Influence on Gandhi: The Evolution of Civil DisobedienceRajdeep Bavaliya
Ã˝
Dive into the powerful journey from Thoreau’s 19th‑century essay to Gandhi’s mass movement, and discover how one man’s moral stand became the backbone of nonviolent resistance worldwide. Learn how conscience met strategy to spark revolutions, and why their legacy still inspires today’s social justice warriors. Uncover the evolution of civil disobedience. Don’t forget to like, share, and follow for more deep dives into the ideas that changed the world.
M.A. Sem - 2 | Presentation
Presentation Season - 2
Paper - 108: The American Literature
Submitted Date: April 2, 2025
Paper Name: The American Literature
Topic: Thoreau’s Influence on Gandhi: The Evolution of Civil Disobedience
[Please copy the link and paste it into any web browser to access the content.]
Video Link: https://youtu.be/HXeq6utg7iQ
For a more in-depth discussion of this presentation, please visit the full blog post at the following link: https://rajdeepbavaliya2.blogspot.com/2025/04/thoreau-s-influence-on-gandhi-the-evolution-of-civil-disobedience.html
Please visit this blog to explore additional presentations from this season:
Hashtags:
#CivilDisobedience #ThoreauToGandhi #NonviolentResistance #Satyagraha #Transcendentalism #SocialJustice #HistoryUncovered #GandhiLegacy #ThoreauInfluence #PeacefulProtest
Keyword Tags:
civil disobedience, Thoreau, Gandhi, Satyagraha, nonviolent protest, transcendentalism, moral resistance, Gandhi Thoreau connection, social change, political philosophyPaper 107 | From Watchdog to Lapdog: Ishiguro’s Fiction and the Rise of “Godi...
Paper 107 | From Watchdog to Lapdog: Ishiguro’s Fiction and the Rise of “Godi...Rajdeep Bavaliya
Ã˝
Dive into a captivating analysis where Kazuo Ishiguro’s nuanced fiction meets the stark realities of post‑2014 Indian journalism. Uncover how “Godi Media” turned from watchdog to lapdog, echoing the moral compromises of Ishiguro’s protagonists. We’ll draw parallels between restrained narrative silences and sensationalist headlines—are our media heroes or traitors? Don’t forget to follow for more deep dives!
M.A. Sem - 2 | Presentation
Presentation Season - 2
Paper - 107: The Twentieth Century Literature: From World War II to the End of the Century
Submitted Date: April 4, 2025
Paper Name: The Twentieth Century Literature: From World War II to the End of the Century
Topic: From Watchdog to Lapdog: Ishiguro’s Fiction and the Rise of “Godi Media” in Post-2014 Indian Journalism
[Please copy the link and paste it into any web browser to access the content.]
Video Link: https://youtu.be/kIEqwzhHJ54
For a more in-depth discussion of this presentation, please visit the full blog post at the following link: https://rajdeepbavaliya2.blogspot.com/2025/04/from-watchdog-to-lapdog-ishiguro-s-fiction-and-the-rise-of-godi-media-in-post-2014-indian-journalism.html
Please visit this blog to explore additional presentations from this season:
Hashtags:
#GodiMedia #Ishiguro #MediaEthics #WatchdogVsLapdog #IndianJournalism #PressFreedom #LiteraryCritique #AnArtistOfTheFloatingWorld #MediaCapture #KazuoIshiguro
Keyword Tags:
Godi Media, Ishiguro fiction, post-2014 Indian journalism, media capture, Kazuo Ishiguro analysis, watchdog to lapdog, press freedom India, media ethics, literature and media, An Artist of the Floating WorldICT-8-Module-REVISED-K-10-CURRICULUM.pdf
ICT-8-Module-REVISED-K-10-CURRICULUM.pdfpenafloridaarlyn
Ã˝
In this module, you will discover how digital tools, systems, and platforms empower people, businesses, and communities in the modern world. As 21st-century learners, you are part of a generation that lives and learns in a digital environment. This module is designed to guide you in exploring how ICT serves as a powerful tool—not only for communication but also for innovation, entrepreneurship, and responsible citizenship. Throughout this learning material, you will examine how ICT is used in real-world scenarios such as online marketing, digital citizenship, and legal and ethical issues in technology use. You’ll gain practical knowledge and skills, from creating websites and managing e-commerce platforms, to analyzing data and practicing safe and responsible behavior online.
By engaging with the lessons, activities, and performance tasks in this module, you will become more than just a technology user—you will be a responsible, informed, and empowered digital citizen ready to thrive in today’s interconnected world.
Let’s begin this journey and unlock the full potential of ICT in your everyday life!
FIRST DAY HIGH orientation for mapeh subject in grade 10.pptx
FIRST DAY HIGH orientation for mapeh subject in grade 10.pptxGlysdiEelesor1
Ã˝
basic orientation of the first day highRevista digital preescolar en transformación
Revista digital preescolar en transformaciónguerragallardo26
Ã˝
EVOLUCIÓN DEL CONTENIDO DE LA EVALUACIÓN DE LOS RECURSOS Y DE LA FORMACIÓN DE LOS DOCENTESABCs of Bookkeeping for Nonprofits TechSoup.pdf
ABCs of Bookkeeping for Nonprofits TechSoup.pdfTechSoup
Ã˝
Accounting can be hard enough if you haven’t studied it in school. Nonprofit accounting is actually very different and more challenging still.
Need help? Join Nonprofit CPA and QuickBooks expert Gregg Bossen in this first-time webinar and learn the ABCs of keeping books for a nonprofit organization.
Key takeaways
* What accounting is and how it works
* How to read a financial statement
* What financial statements should be given to the board each month
* What three things nonprofits are required to track
What features to use in QuickBooks to track programs and grantsHow to Configure Vendor Management in Lunch App of Odoo 18
How to Configure Vendor Management in Lunch App of Odoo 18Celine George
Ã˝
The Vendor management in the Lunch app of Odoo 18 is the central hub for managing all aspects of the restaurants or caterers that provide food for your employees. Paper 107 | From Watchdog to Lapdog: Ishiguro’s Fiction and the Rise of “Godi...
Paper 107 | From Watchdog to Lapdog: Ishiguro’s Fiction and the Rise of “Godi...Rajdeep Bavaliya
Ã˝
Ad
writing ionic compounds in chemistry.pptx
- 1. 1 Aim: How do we write the chemical formula of an ionic compound given the chemical name
- 2. Binary Compound  Binary Compounds are composed of two elements bonded together  One METAL Ion and One NONMETAL Ion for Ionic Compound  Metal atoms lose electrons to form positively charged ions. Some metals form one ion, while others (usually transition metals) can form multiple ions.  Nonmetal atoms gain electrons to form negatively charged ions. Nonmetals form only one negative ion.  General naming format for binary ionic compounds “metal ion name + nonmetal ion name”
- 3. 3 Naming Ionic compounds with metals that have only one oxidation state
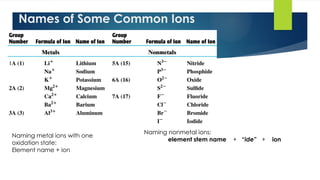
- 4. 4 Names of Some Common Ions Naming metal ions with one oxidation state: Element name + ion Naming nonmetal ions: element stem name + “ide” + ion
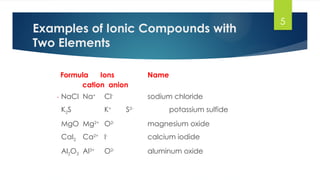
- 5. 5 Formula Ions Name cation anion NaCl Na+ Cl- sodium chloride K2S K+ S2- potassium sulfide MgO Mg2+ O2- magnesium oxide CaI2 Ca2+ I- calcium iodide Al2O3 Al3+ O2- aluminum oxide Examples of Ionic Compounds with Two Elements
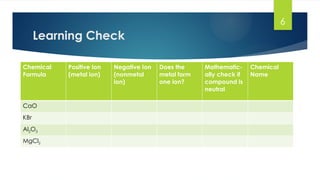
- 6. 6 Learning Check Chemical Formula Positive Ion (metal ion) Negative Ion (nonmetal ion) Does the metal form one ion? Mathematic- ally check if compound is neutral Chemical Name CaO KBr Al2O3 MgCl2
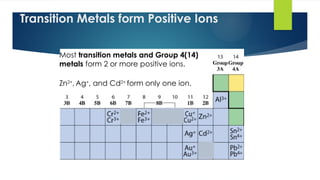
- 7. 7 Transition Metals form Positive Ions Most transition metals and Group 4(14) metals form 2 or more positive ions. Zn2+ , Ag+ , and Cd2+ form only one ion.
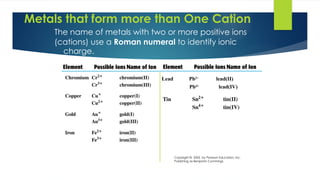
- 8. 8 Metals that form more than One Cation The name of metals with two or more positive ions (cations) use a Roman numeral to identify ionic charge. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings Lead Pb2+ lead(II) Pb4+ lead(IV)
- 9. 9 Naming Variable Charge Metals Transition metals with two or more different ions use a Roman numeral after the name of the metal to indicate ionic charge.
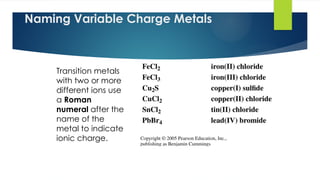
- 10. 10 Naming Ionic Compounds with Variable Charge Metals
- 11. 11 Naming FeCl2 To name FeCl2 1. Determine the charge of the cation using the charge of the anion (Cl- ). Fe ion + 2 Cl- = Fe ion + 2 (-1) = 0 Fe ion = 2+ 2. Name the cation by the element name and add a Roman numeral in parenthesis to show its charge. Fe2+ = iron(II) 3. Write the anion with an ide ending. FeCl2 = iron(II) chloride
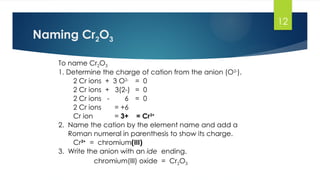
- 12. 12 Naming Cr2O3 To name Cr2O3 1. Determine the charge of cation from the anion (O2- ). 2 Cr ions + 3 O2- = 0 2 Cr ions + 3(2-) = 0 2 Cr ions - 6 = 0 2 Cr ions = +6 Cr ion = 3+ = Cr3+ 2. Name the cation by the element name and add a Roman numeral in parenthesis to show its charge. Cr3+ = chromium(III) 3. Write the anion with an ide ending. chromium(III) oxide = Cr2O3
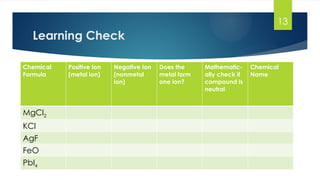
- 13. 13 Learning Check Chemical Formula Positive Ion (metal ion) Negative Ion (nonmetal ion) Does the metal form one ion? Mathematic- ally check if compound is neutral Chemical Name MgCl2 KCl AgF FeO PbI4
- 14. 14 1. Determine if the metal is a transition metal. 2. If Transition Metal, Determine the proper oxidation number to use. (Use nonmetal to help you determine) 3. Write down the name of the metal. (use roman numerals to show oxidation state for transition metals) 4. Write the name of the non-metal, changing the ending to ide Ex: Oxide, Fluoride, Bromide, Chloride, Nitride Summary Steps to naming Binary Ionic Compounds