Bio marker introduction
- 2. Introduction 2 ï Definition: The US FDA (2001)defines a biomarker as a characteristic i.e. objectively measured & evaluated as an indicator of normal biologic, pathogenic or pharmacologic responses to therapeutic intervention.
- 3. ï WHO (2010) ï Biomarkers includes- âAlmost any measurement reflecting an interaction between a biological system and a potential hazard, which may be chemical, physical, or biological; The measured response may be functional and physiological, biochemical at the cellular level or a molecular interaction.â ï Ex : Pulse and blood pressure through basic chemistries to more complex laboratory tests of blood and other tissues.
- 7. Biomarker & Diagnosis Ideal Marker for diagnosis âĒShould have great sensitivity (>0.9), specificity (>0.9), and accuracy in reflecting total disease burden. âĒA tumor marker should also be prognostic of outcome and treatment. Samples for biomarker detection âĒBlood, urine, or other body fluids samples âĒTissue samples.
- 8. Biomarker for Screening ï The marker must be highly specific, minimize false positive and negative ï The marker must be able to clearly reflect the different stages of the disease (early) ï The marker must be easily detected without complicated medical procedures. ï The disease markers released to serum and urine are good targets for application of early screening. ï The method for screening should be cost effective.
- 9. Biomarker Detection of biomarker â diagnosis Self properties, e.g enzymatic activities, Antibodies, IHC, ELISA. Detection of biomarker âĒQuantitative a link between quantity of the marker and disease âĒQualitative a link between exist of a marker and disease.
- 10. Quantitative Approaches Stable Isotope Labeling methods âĒadds heavy isotopes to one sample so chemically identical compounds are mass shifted âĒadded to the peptides/proteins using reactive groups âĒadded to the proteins in vivo using heavy amino acids âĒcan be multiplexed Label free methods âĒextracted ion chromatograms âĒspectral counting
- 11. Validation of Biomaker ï Accuracy (agreement with a reference) ï Precision (repeatability, reproducibility) ï Limit of Detection (sensitivity) ï Interference, Cross-reactivity (specificity) ï Sample preparation / conditions ï Performance around the cut-off ï Potential for carryover, cross-hybridization.
- 12. Biomarker Uses ï Diagnosis, in symptomatic patients ï Early detection (screening), enabling intervention at an earlier and potentially more curable stage than under usual clinical diagnostic conditions ï Monitoring of disease response during therapy, with potential for adjusting level of intervention (e.g. dose) on a dynamic and personal basis ï Risk assessment, leading to preventive interventions for those at sufficient risk ï Prognosis, allowing for more (less) aggressive therapy for patients with worse (better) prognosis ï Prediction. E.g., predicts safety, efficacy (PK/PD) of a specific therapy, thereby providing guidance in selecting it for patients or tailoring its dose. Last three are attempts to predict the future.
- 13. Advantages Disadvantages Objective assessment Timing is critical Precision of measurement Expensive (cost for analysis) Reliable; validity can be established Storage (longevity of samples) Less biased than questionnaires Laboratory errors Disease mechanisms often studied Normal range difficult to establish Homogeneity of risk or disease Ethical responsibility Biomarker
- 15. Ideal biomarker for sepsis ï Sensitive and specific for bacterial infection . ï Single reliable cut-off value. ï Unaffected by acute illness or chronic disease. ï Level correlates with severity and mortality. ï Short half-life. ï Inexpensive and timely result (<1hr). ï Ex . Procalcitonin ,CRP,aptt,TNF, IL
- 16. ï The potential role of biomarkers for diagnosis remains undefined. ï Recomendation ï Use of low procalcitonin levels or similar biomarkers to assist the clinician in the discontinuation of empiric antibiotics in patients who initially appeared septic, but have no subsequent evidence of infection (grade 2C). International Guidelines for Management of Severe Sepsis and Septic Shock: 2013
- 18. Malaria diagnosis serological biomarker ï Rapid detection tests (RDTs) - immunochromatography assay. ïPlasmodial lactate dehydrogenase (pLDH). ïHistidine-rich protein II (HRP II). ï Published sensitivities of RDTs for P. falciparum range from comparable to those of good field microscopy (> 90% at 100â500 parasites/Ξl of blood) to very poor (40â 50%) for some widely used products. Priyamvada Jain et al; Potential Biomarkers and Their Applications for Rapid and Reliable Detection of Malaria. 852645, 2014, 1-20. The use of rapid diagnostic tests. Geneva, Roll Back Malaria, WHO Regional Office for the Western Pacific and UNDP/World Bank/WHO/UNICEF Special Programme for Research
- 19. ï WHO maintains a list of RDT manufacturers with ISO 13485:2003 certification. Advantages ï Rapid diagnosis. ï Fewer requirement of trained and skilled personal. Disadvantages ï Unpredictable sensitivity. ï Inability to differentiate between new infection and recently treated infection as few antibodies (HRP2) persist for 2 to 3 weeks.
- 22. TB pleural effusion (ADA) ï§ Diagnosis of TB pleural effusion is a challenging task. None of the diagnostic modalities available till date are able to diagnose TB pleural effusion as ï§ So ADA is being used as a biomarker in diagnosing TB pleural effusion . ï§ It is a quantitative biomarker produced from T Lymphocytes .
- 26. LAM ï Detection of the Mycobacterium tuberculosis cell wall antigen lipoarabinomannan (LAM) in urine permits diagnoses of tuberculosis (TB). ï This can be achieved at the point-of-care within just 30 minutes using the Determine TB-LAM, which is a commercially available, lateral-flow urine âstrip testâ assay. ï Currently this test is validated in africa in HIV positive patients . ï Sensitivity and specificity are low in compared to the Xpert MTB/RIF assay.
- 27. Advantages ï simplicity of use, ï lack of instrumentation, ï speed of use with results available after 25 minutes ï Low cost (initially marketed at $3.50 per test) ï Implemented at the point-of-care. ï This is currently used in patients enrolled for ART treatment as a rapid test to rule out dissemenated TB.
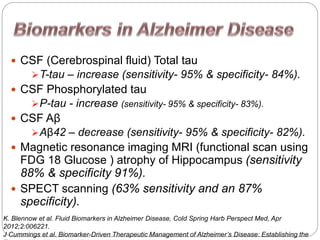
- 28. ï CSF (Cerebrospinal fluid) Total tau ïT-tau â increase (sensitivity- 95% & specificity- 84%). ï CSF Phosphorylated tau ïP-tau - increase (sensitivity- 95% & specificity- 83%). ï CSF AÎē ïAÎē42 â decrease (sensitivity- 95% & specificity- 82%). ï Magnetic resonance imaging MRI (functional scan using FDG 18 Glucose ) atrophy of Hippocampus (sensitivity 88% & specificity 91%). ï SPECT scanning (63% sensitivity and an 87% specificity). K. Blennow et al. Fluid Biomarkers in Alzheimer Disease, Cold Spring Harb Perspect Med, Apr 2012;2:006221. J Cummings et al. Biomarker-Driven Therapeutic Management of Alzheimerâs Disease: Establishing the
- 29. ï CSF Amyloid Îē and tau. ï Neuromelanin antibodies. ï PET biomarkers include [18F]-DOPA for estimating dopaminergic neurotransmission. ï Reduced brain regional N-acetyl-aspartate using magnetic resonance spectroscopy. ï Îą-Synculein index & Charnoly body.
Editor's Notes
- The utility of procalcitonin levels or other biomarkers (such as CRP) to discriminate the acute inflammatory pattern of sepsis from other causes of generalized inflammation (eg, postoperative, other forms of shock) has not been demonstrated
- Currently who recomends use of



























![ï CSF Amyloid Îē and tau.
ï Neuromelanin antibodies.
ï PET biomarkers include [18F]-DOPA for estimating
dopaminergic neurotransmission.
ï Reduced brain regional N-acetyl-aspartate using
magnetic resonance spectroscopy.
ï Îą-Synculein index & Charnoly body.](https://image.slidesharecdn.com/biomarkerintroduction-150513135533-lva1-app6891/85/Bio-marker-introduction-29-320.jpg)
