P phosphorus
- 1. Phosphorus Aidan Hardekopf
- 2. General info li ck m e C âĒ Atomic number: 15 âĒ ClassiïŽcation: Nonmetal âĒ Symbol: P Red phosphorus powder. âĒ Amount of protons/neutrons/electrons: 15/16/15 respectively âĒ Melting and boiling points: 44.2 and 280.5 degrees Celsius âĒ Colored in red, white, black and a rare violet. âĒ Natural phase of matter: solid âĒ Atomic mass: 30.973...
- 3. History of phosphorus âĒ Name origin: In Greek, "phÃīs" is light and "phoros" is bearer. Therefore, phosphorous is the "light bearer. âĒ Discovered by Hennig Brand in 1669 in attempt to create the Philosopher's Stone. The stone is said to turn all solids into gold (which is absolutely unreal.) Brand wanted to keep the element a secret, until he told a few other scientists. Even Robert Boyle got his hands on the element. It wasn't until the late 18th century that it was discovered for use in fertilizer. âĒ Phosphorus is known to be found in DNA and RNA ever since the genetic code's existence. Depiction of r e! Brand he discovering li ck phosphorus C
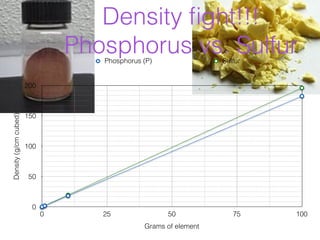
- 4. Density ïŽght!!! Phosphorus vs. Sulfur Phosphorus (P) Sulfur 200 Density (g/cm cubed) 150 100 50 0 0 25 50 75 100 Grams of element
- 5. Uses for phosphorus Red phosphorus is used in match heads. You can see the texture of a White phosphorus is match head next to the matches. ! used in some Fertilizer; explosives, including phosphorus is rockets. This caused known for being an uproar because of essential to safety concerns. Watch a video of a DNA and to a white phosphorus explosion lesser extent fertilizer.
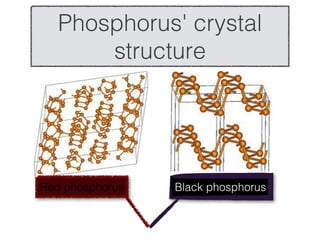
- 6. Phosphorus' crystal structure Red phosphorus Black phosphorus
- 7. Did you know...? âĒ When Hennig Brand discovered phosphorus, he made it by boiling remains of boiled urine. âĒ Hamburg, the town this element was discovered in, was bombed with caustic white phosphorus explosives. The city erupted in ïŽames. Background: Hamburg in the 19th century.
- 8. The Element Boxes It became apparent that elements became the subject of interest in NPMM. Therefore, each eighth grade student decided to create an element box, containing basic information about each element. Each one comes with a Keynote presentation and video (hidden in a QR code) of each element. Mine is about phosphorus, an element you have learned has an abundance of uses, an odd history and one heck of an explosion. Each box also has artifacts, which symbolize what the element is used for.