Veled is megtÃķrtÃĐnhet!
Download as pptx, pdf0 likes457 views
BÅąnmegelÅzÃĐs ÃĐs baleset-megelÅzÃĐs kapcsolata. A peer-to-peer mÃģdszertan alkalmazÃĄsa a tudatos kÃķzlekedÃĐsre nevelÃĐsben.
1 of 10
Download to read offline








Ad
Recommended
Haile Middle School: Properties
Haile Middle School: PropertiesHannah5460
Ėý
The document discusses various properties of mathematics including the commutative property, distributive property, identity property, associative property, multiplication by zero property, and multiplication by -1 property. Examples are provided to illustrate each property, such as how the order of addition or multiplication does not change the sum or product under the commutative property. Practice problems are also included for the reader to identify which property each example demonstrates.Chapter 6 Atoms & their interactions
Chapter 6 Atoms & their interactionsjaguilar
Ėý
The document discusses the basics of chemistry as it relates to life, including the structure of elements and atoms, chemical bonds, water and diffusion, and life substances such as carbohydrates, lipids, proteins, and nucleic acids. It explains that carbon can form multiple bonds which allows it to link to other carbon atoms, forming large and complex molecules essential for life. The document provides foundational chemistry concepts to understand the compounds that make up living organisms.L4
L4sgl9vp
Ėý
The document discusses radioactive decay, uses of radiation in medicine, and calculating radiation dose. It also mentions the principle of keeping radiation risks as low as reasonably achievable, known by the acronym ALARA, which is enforced by law when using radiation.Atoms:
Atoms: Myzel Fevie Baltazar
Ėý
Matter is made up of atoms, which are the smallest particles that make up matter. Atoms contain protons, neutrons, and electrons. Protons and neutrons are located in the center of the atom, called the nucleus, while electrons orbit around the nucleus. Scientists like Democritus, Dalton, Thompson, Rutherford, and Bohr contributed theories to our modern understanding of atomic structure, such as electrons orbiting the nucleus in specific energy levels.Atoms are amazing!12
Atoms are amazing!12jsetsma
Ėý
Atoms are the basic building blocks that make up all matter. They are incredibly small, with a single cubic centimeter containing trillions of air molecules. Atoms are mostly empty space, with electrons orbiting a tiny, dense nucleus at great distances comparable to the size of a stadium. Elements are pure substances composed of only one type of atom that can be characterized by their atomic number and mass.Describing motion
Describing motionphy201516
Ėý
This document discusses distance-time graphs and their relationship to speed, velocity, and acceleration. It contains the following key points:
1. A diagonal line on a distance-time graph indicates constant speed. The steeper the line, the greater the speed.
2. Acceleration occurs when velocity increases, decreases, or changes direction. It is the rate of change in velocity over time.
3. The area under a speed-time graph represents the total distance travelled. The gradient of the line equals the speed. Constant speed is shown by a straight diagonal line where area equals distance.Atoms â The Inside Story
Atoms â The Inside Storyamandayoung313
Ėý
Atoms are the smallest building blocks of matter and are composed of protons, neutrons, and electrons. Protons and neutrons are located in the central nucleus, while electrons occupy the space surrounding the nucleus in an electron cloud. Different atomic models were developed over centuries before scientists concluded that protons and neutrons are in the nucleus, with electrons orbiting in the electron cloud outside the nucleus.Chapter 8
Chapter 8kau_deanship of e-learning and distance education
Ėý
This document discusses periodic trends in atomic and ionic properties, including:
- Atomic and ionic radii decrease across a period as effective nuclear charge increases. Radii increase down a group as principal quantum number increases.
- Ionization energies generally increase across a period as it becomes more difficult to remove electrons. Exceptions include group 2A and 5A having higher energies than 3A and 6A respectively within periods.
- Cations have smaller radii than their parent atoms as electrons are removed. Anions have larger radii as more electrons are gained. Isotectronic ions with more protons have smaller radii.Inside the Atom~Notes
Inside the Atom~Notesduncanpatti
Ėý
The document discusses the structure and composition of atoms. It can be summarized as follows:
1) Atoms have a small, dense nucleus containing protons and neutrons, surrounded by an electron cloud where electrons are found.
2) The number of protons determines the element, and protons plus neutrons equals the mass number. Electrons equal protons for neutral atoms.
3) Atoms can gain or lose electrons to become ions with a positive or negative charge.
4) Isotopes of the same element have different numbers of neutrons but the same number of protons.WAVE AND ITS PROPERTIES
WAVE AND ITS PROPERTIESPaul Nicko
Ėý
The document defines key terms related to waves, including:
- Crest, trough, amplitude, wavelength, frequency, period, wave speed, wavelength, loudness, intensity, quality, and pitch. It also describes transverse waves, where particles vibrate perpendicular to the wave, and longitudinal waves, where particles vibrate parallel to the wave. Reflection and diffraction of waves are also discussed.Types of waves
Types of wavesjn05
Ėý
The document discusses two types of mechanical waves: transverse waves and longitudinal waves. Transverse waves involve a medium that moves perpendicular to the direction of energy transfer, while longitudinal waves involve a medium that moves parallel to the direction of energy transfer. Longitudinal waves are characterized by regions of compression and rarefaction along the direction of the wave. Both transverse and longitudinal waves transfer energy through a medium and can be described by their amplitude, wavelength, period, frequency, and velocity.Waves Basics
Waves BasicsDaniel McClelland
Ėý
This document provides an overview of wave motion, including the following key points:
- There are two types of wave motion: longitudinal waves, where particle motion is parallel to the direction of energy transfer, and transverse waves, where particle motion is perpendicular. Sound waves are longitudinal while light waves are transverse.
- Key wave properties are defined, including wavelength, frequency, amplitude, and speed. The wave equation relating these properties is presented.
- Reflection and refraction of waves is demonstrated using wavefront diagrams, showing how waves change direction at boundaries between mediums. Refraction occurs when waves move from deep to shallow water, changing the wavelength.Wave Motion
Wave MotionKristie Barber
Ėý
The document discusses different types of waves, including transverse waves and longitudinal waves. It defines key wave properties such as amplitude, wavelength, period, and frequency. It explains that amplitude measures intensity, wavelength is the distance between crests, period is the time for one full wave cycle, and frequency is the number of cycles per unit time. The document also defines transverse wave properties like crests and troughs and longitudinal wave properties like compression and rarefaction.waves
wavesDiana Liu
Ėý
The document discusses different types of waves including transverse waves, where the displacement is perpendicular to the direction of motion, and longitudinal waves, where the displacement is parallel. It defines key wave properties like speed, frequency, wavelength, and how speed equals frequency multiplied by wavelength. It describes constructive and destructive interference from crests and troughs combining or canceling. It lists tsunamis as being caused by earthquakes, landslides, and volcanoes and mentions water circulation and ocean waves.Two types of_waves
Two types of_waveskoniasunset
Ėý
Waves transfer energy through a medium by causing a disturbance that moves through the medium without the medium itself moving; there are mechanical waves which need a medium and electromagnetic waves which do not. Mechanical waves include sound waves and ocean waves, while electromagnetic waves include visible light, radio waves, x-rays and more, and all waves can be characterized by their wavelength, frequency, amplitude, and speed which depends on the medium.Waves Presentation
Waves Presentationspartahp
Ėý
The document discusses different parts of waves including the crest, trough and wavelength. It defines amplitude as loudness and frequency as pitch measured in Hertz. It explains that sound waves use energy to move and we hear them through our ears when someone talks, though we can't see the sound waves. When combining waves, high pressures cancel low pressures while two high or low pressures reinforce each other.General Wave Properties
General Wave Propertiesmeenng
Ėý
This document discusses different types of waves including transverse waves, longitudinal waves, and properties of waves such as amplitude, wavelength, frequency, and speed. It defines a wave as a spread of disturbance and notes that waves can transfer energy. It provides examples of transverse waves like water waves and electromagnetic waves, and examples of longitudinal waves like sound waves. It also defines key wave terms and properties and includes the wave equation relating wavelength, frequency, and speed.Chapter 2 the structure of the atom
Chapter 2 the structure of the atomLing Leon
Ėý
The document discusses the structure of atoms and isotopes. It begins by defining matter and the particle theory of matter. It then explains that atoms are made up of protons, neutrons and electrons. The atomic structure of various elements is discussed through their electron configurations. Isotopes are then introduced as atoms of the same element that have the same number of protons but different numbers of neutrons. Examples of isotopes including hydrogen and oxygen isotopes are provided.Struture of an atom
Struture of an atomNiharika Pande
Ėý
This document provides an outline for a presentation on the history and structure of atoms. It begins with the early ideas of Democritus and Thomson, then describes the atomic models of Rutherford, Bohr, and others. The structure of the atom is explained, including the nucleus, protons, neutrons, and electrons. Isotopes and isobars are also introduced. The presentation would provide details on the key scientists and experiments that led to the current understanding of atoms as tiny particles consisting of a nucleus surrounded by electrons.Structure of atom ppt
Structure of atom pptlekshmisg91
Ėý
Atoms are the basic building blocks of all matter. John Dalton proposed that atoms are indivisible and identical for each element. Rutherford discovered that atoms have a small, dense nucleus at the center surrounded by electrons. Niels Bohr modeled atoms with electrons orbiting the nucleus in fixed energy levels or shells. The nucleus contains protons and neutrons, while electrons orbit outside. The number of protons determines the element, and protons and neutrons together equal the mass number.Science Grade 8 Teachers Manual
Science Grade 8 Teachers ManualOrland Marc Enquig
Ėý
This unit discusses force, motion, and energy. It has six modules that describe energy transfer at both the macroscopic and particle levels. Module 1 focuses on how unbalanced forces cause changes in motion. Module 2 explains how force can do work and transfer energy. The unit aims to develop students' understanding that energy is transmitted through various means and can cause changes in objects. Most topics are dealt with qualitatively to provide a basic understanding of concepts.MinÅsÃĐgmenedzsment - TechnolÃģgiÃĄval a pedagÃģgusok tehermenetesÃtÃĐsÃĐÃĐrt
MinÅsÃĐgmenedzsment - TechnolÃģgiÃĄval a pedagÃģgusok tehermenetesÃtÃĐsÃĐÃĐrtITStudy Ltd.
Ėý
Az OpenQAsS projektrÅl a Tempus KÃķzalapÃtvÃĄny PÃĄlyÃĄzati pavilon c. kiadvÃĄnyÃĄnak 1018 tavaszi szÃĄmÃĄban megjelent cikk.Otp mentorirsz kerÃĐkl_2012
Otp mentorirsz kerÃĐkl_2012POZITEAM
Ėý
KerÃĐk LÃĄszlÃģ elÅadÃĄsa az OTP mentori rendszerÃĐrÅl. (TudÃĄsmenedzsment trÃĐning, 2012. november 20-21.)Auchan e learning koncepcio-bemutatasa20091116
Auchan e learning koncepcio-bemutatasa20091116zsolt jenei
Ėý
eLearning koncepciÃģ bemutatÃĄsa 2010-beniGEN Debrecen - a kÃĐpzÃĐs bemutatÃĄsa
iGEN Debrecen - a kÃĐpzÃĐs bemutatÃĄsaMÃĄtÃĐ Rab
Ėý
InnovÃĄtor, startupper ÃĐs fiatal vÃĄllalkozÃģ kÃĐpzÃĐs Debrecenben! RÃĐszletek: www.igendebrecen.huAz ÃķregedÅ munkaerÅ tudÃĄsÃĄnak megÅrzÃĐse (RÃĄba esettanulmÃĄny)
Az ÃķregedÅ munkaerÅ tudÃĄsÃĄnak megÅrzÃĐse (RÃĄba esettanulmÃĄny)Tibor Gyulay
Ėý
Dr. Bencsik Andrea elÅadÃĄsa a KM EXPERT 2017. mÃĄrcius 22-i tudÃĄsmenedzsment mÚhelyÃĐnLeonardo Partnership projekt bemutatÃģ 2014
Leonardo Partnership projekt bemutatÃģ 2014CEIPES HUNGARY NGO
Ėý
Leonardo Partnership Projekt presentationMore Related Content
Viewers also liked (13)
Inside the Atom~Notes
Inside the Atom~Notesduncanpatti
Ėý
The document discusses the structure and composition of atoms. It can be summarized as follows:
1) Atoms have a small, dense nucleus containing protons and neutrons, surrounded by an electron cloud where electrons are found.
2) The number of protons determines the element, and protons plus neutrons equals the mass number. Electrons equal protons for neutral atoms.
3) Atoms can gain or lose electrons to become ions with a positive or negative charge.
4) Isotopes of the same element have different numbers of neutrons but the same number of protons.WAVE AND ITS PROPERTIES
WAVE AND ITS PROPERTIESPaul Nicko
Ėý
The document defines key terms related to waves, including:
- Crest, trough, amplitude, wavelength, frequency, period, wave speed, wavelength, loudness, intensity, quality, and pitch. It also describes transverse waves, where particles vibrate perpendicular to the wave, and longitudinal waves, where particles vibrate parallel to the wave. Reflection and diffraction of waves are also discussed.Types of waves
Types of wavesjn05
Ėý
The document discusses two types of mechanical waves: transverse waves and longitudinal waves. Transverse waves involve a medium that moves perpendicular to the direction of energy transfer, while longitudinal waves involve a medium that moves parallel to the direction of energy transfer. Longitudinal waves are characterized by regions of compression and rarefaction along the direction of the wave. Both transverse and longitudinal waves transfer energy through a medium and can be described by their amplitude, wavelength, period, frequency, and velocity.Waves Basics
Waves BasicsDaniel McClelland
Ėý
This document provides an overview of wave motion, including the following key points:
- There are two types of wave motion: longitudinal waves, where particle motion is parallel to the direction of energy transfer, and transverse waves, where particle motion is perpendicular. Sound waves are longitudinal while light waves are transverse.
- Key wave properties are defined, including wavelength, frequency, amplitude, and speed. The wave equation relating these properties is presented.
- Reflection and refraction of waves is demonstrated using wavefront diagrams, showing how waves change direction at boundaries between mediums. Refraction occurs when waves move from deep to shallow water, changing the wavelength.Wave Motion
Wave MotionKristie Barber
Ėý
The document discusses different types of waves, including transverse waves and longitudinal waves. It defines key wave properties such as amplitude, wavelength, period, and frequency. It explains that amplitude measures intensity, wavelength is the distance between crests, period is the time for one full wave cycle, and frequency is the number of cycles per unit time. The document also defines transverse wave properties like crests and troughs and longitudinal wave properties like compression and rarefaction.waves
wavesDiana Liu
Ėý
The document discusses different types of waves including transverse waves, where the displacement is perpendicular to the direction of motion, and longitudinal waves, where the displacement is parallel. It defines key wave properties like speed, frequency, wavelength, and how speed equals frequency multiplied by wavelength. It describes constructive and destructive interference from crests and troughs combining or canceling. It lists tsunamis as being caused by earthquakes, landslides, and volcanoes and mentions water circulation and ocean waves.Two types of_waves
Two types of_waveskoniasunset
Ėý
Waves transfer energy through a medium by causing a disturbance that moves through the medium without the medium itself moving; there are mechanical waves which need a medium and electromagnetic waves which do not. Mechanical waves include sound waves and ocean waves, while electromagnetic waves include visible light, radio waves, x-rays and more, and all waves can be characterized by their wavelength, frequency, amplitude, and speed which depends on the medium.Waves Presentation
Waves Presentationspartahp
Ėý
The document discusses different parts of waves including the crest, trough and wavelength. It defines amplitude as loudness and frequency as pitch measured in Hertz. It explains that sound waves use energy to move and we hear them through our ears when someone talks, though we can't see the sound waves. When combining waves, high pressures cancel low pressures while two high or low pressures reinforce each other.General Wave Properties
General Wave Propertiesmeenng
Ėý
This document discusses different types of waves including transverse waves, longitudinal waves, and properties of waves such as amplitude, wavelength, frequency, and speed. It defines a wave as a spread of disturbance and notes that waves can transfer energy. It provides examples of transverse waves like water waves and electromagnetic waves, and examples of longitudinal waves like sound waves. It also defines key wave terms and properties and includes the wave equation relating wavelength, frequency, and speed.Chapter 2 the structure of the atom
Chapter 2 the structure of the atomLing Leon
Ėý
The document discusses the structure of atoms and isotopes. It begins by defining matter and the particle theory of matter. It then explains that atoms are made up of protons, neutrons and electrons. The atomic structure of various elements is discussed through their electron configurations. Isotopes are then introduced as atoms of the same element that have the same number of protons but different numbers of neutrons. Examples of isotopes including hydrogen and oxygen isotopes are provided.Struture of an atom
Struture of an atomNiharika Pande
Ėý
This document provides an outline for a presentation on the history and structure of atoms. It begins with the early ideas of Democritus and Thomson, then describes the atomic models of Rutherford, Bohr, and others. The structure of the atom is explained, including the nucleus, protons, neutrons, and electrons. Isotopes and isobars are also introduced. The presentation would provide details on the key scientists and experiments that led to the current understanding of atoms as tiny particles consisting of a nucleus surrounded by electrons.Structure of atom ppt
Structure of atom pptlekshmisg91
Ėý
Atoms are the basic building blocks of all matter. John Dalton proposed that atoms are indivisible and identical for each element. Rutherford discovered that atoms have a small, dense nucleus at the center surrounded by electrons. Niels Bohr modeled atoms with electrons orbiting the nucleus in fixed energy levels or shells. The nucleus contains protons and neutrons, while electrons orbit outside. The number of protons determines the element, and protons and neutrons together equal the mass number.Science Grade 8 Teachers Manual
Science Grade 8 Teachers ManualOrland Marc Enquig
Ėý
This unit discusses force, motion, and energy. It has six modules that describe energy transfer at both the macroscopic and particle levels. Module 1 focuses on how unbalanced forces cause changes in motion. Module 2 explains how force can do work and transfer energy. The unit aims to develop students' understanding that energy is transmitted through various means and can cause changes in objects. Most topics are dealt with qualitatively to provide a basic understanding of concepts.Similar to Veled is megtÃķrtÃĐnhet! (11)
MinÅsÃĐgmenedzsment - TechnolÃģgiÃĄval a pedagÃģgusok tehermenetesÃtÃĐsÃĐÃĐrt
MinÅsÃĐgmenedzsment - TechnolÃģgiÃĄval a pedagÃģgusok tehermenetesÃtÃĐsÃĐÃĐrtITStudy Ltd.
Ėý
Az OpenQAsS projektrÅl a Tempus KÃķzalapÃtvÃĄny PÃĄlyÃĄzati pavilon c. kiadvÃĄnyÃĄnak 1018 tavaszi szÃĄmÃĄban megjelent cikk.Otp mentorirsz kerÃĐkl_2012
Otp mentorirsz kerÃĐkl_2012POZITEAM
Ėý
KerÃĐk LÃĄszlÃģ elÅadÃĄsa az OTP mentori rendszerÃĐrÅl. (TudÃĄsmenedzsment trÃĐning, 2012. november 20-21.)Auchan e learning koncepcio-bemutatasa20091116
Auchan e learning koncepcio-bemutatasa20091116zsolt jenei
Ėý
eLearning koncepciÃģ bemutatÃĄsa 2010-beniGEN Debrecen - a kÃĐpzÃĐs bemutatÃĄsa
iGEN Debrecen - a kÃĐpzÃĐs bemutatÃĄsaMÃĄtÃĐ Rab
Ėý
InnovÃĄtor, startupper ÃĐs fiatal vÃĄllalkozÃģ kÃĐpzÃĐs Debrecenben! RÃĐszletek: www.igendebrecen.huAz ÃķregedÅ munkaerÅ tudÃĄsÃĄnak megÅrzÃĐse (RÃĄba esettanulmÃĄny)
Az ÃķregedÅ munkaerÅ tudÃĄsÃĄnak megÅrzÃĐse (RÃĄba esettanulmÃĄny)Tibor Gyulay
Ėý
Dr. Bencsik Andrea elÅadÃĄsa a KM EXPERT 2017. mÃĄrcius 22-i tudÃĄsmenedzsment mÚhelyÃĐnLeonardo Partnership projekt bemutatÃģ 2014
Leonardo Partnership projekt bemutatÃģ 2014CEIPES HUNGARY NGO
Ėý
Leonardo Partnership Projekt presentationAd
Veled is megtÃķrtÃĐnhet!
- 1. VELED IS MEGTÃRTÃNHET! BÅąnmegelÅzÃĐs ÃĐs baleset-megelÅzÃĐs KIBÃDI-VARGA LAJOS Okl. KÃķzlekedÃĐsmÃĐrnÃķk, Pr SzakÃĐrtÅ, Premium Relations, lajos.kibedi@prr.hu BÅąnmegelÅzÃĐsi FÃģrum, Budapest, 2013. februÃĄr 13.
- 4. MI A PROBLÃMA? 4
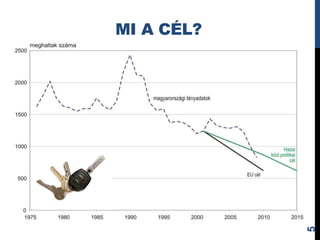
- 5. MI A CÃL? 5
- 6. A PROGRAM CÃLKITÅ°ZÃSEI 1. Balesetek szÃĄmÃĄnak a csÃķkkentÃĐse a 18-25 ÃĐv kÃķzÃķtti korosztÃĄlyban. 2. Fiatal vezetÅk kockÃĄzatvÃĄllalÃģ magatartÃĄsÃĄnak megvÃĄltoztatÃĄsa. 3. Egy hatÃĐkony gyakorlati oktatÃĄsi modul fenntarthatÃģsÃĄgÃĄnak biztosÃtÃĄsa (kÃķzÃĐpiskolÃĄban, autÃģsiskolÃĄban). 6
- 7. A FOGLALKOZÃSOKRÃL âĒ PM (Peer Mentor), âĒ HasonlÃģ korÚ, âĒ HasonlÃģ ÃĐletkÃķrÞlmÃĐnyek kÃķzt ÃĐlÅ, âĒ âEgyenlÅ felek pÃĄrbeszÃĐdeâ âĒ ÃlmÃĐnyszerÅą, ÃĐletszerÅą, âĒ InteraktÃv, âĒ Nem elrettentÅ (!), âĒ Szakemberek jelenlÃĐte (pszicholÃģgus, oktatÃģ). 7
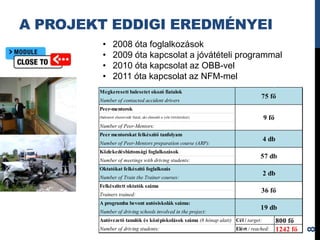
- 8. A PROJEKT EDDIGI EREDMÃNYEI âĒ 2008 Ãģta foglalkozÃĄsok âĒ 2009 Ãģta kapcsolat a jÃģvÃĄtÃĐteli programmal âĒ 2010 Ãģta kapcsolat az OBB-vel âĒ 2011 Ãģta kapcsolat az NFM-mel Megkeresett balesetet okozÃģ fiatalok 75 fÅ Number of contacted accident drivers Peer-mentorok (balesetet elszenvedÅ fiatal, aki elmesÃĐli a vele tÃķrtÃĐnteket) 9 fÅ Number of Peer-Mentors: Peer mentorokat felkÃĐszÃtÅ tanfolyam 4 db Number of Peer-Mentors preparation course (ARP): KÃķzlekedÃĐsbiztonsÃĄgi foglalkozÃĄsok 57 db Number of meetings with driving students: OktatÃģkat felkÃĐszÃtÅ foglalkozÃĄs 2 db Number of Train the Trainer courses: FelkÃĐszÃtett oktatÃģk szÃĄma 36 fÅ Trainers trained: A programba bevont autÃģsiskolÃĄk szÃĄma: 19 db Number of driving schools involved in the project: AutÃģvezetÅ tanulÃģk ÃĐs kÃķzÃĐpiskolÃĄsok szÃĄma (8 hÃģnap alatt) CÃĐl / target: 800 fÅ ElÃĐrt / reached: 1242 fÅ 8 Number of driving students:
- 9. FENNTARTHATÃSÃG? âĒ Szakmai/politikai akarat âĒ PÃĐnzÞgyi fenntarthatÃģsÃĄg âĒ Szervezeti fenntarthatÃģsÃĄg âĒ EgyÞttmÅąkÃķdÃĐs erÅsÃtÃĐse âĒ KIMISZ âĒ BÃrÃģsÃĄgok âĒ ÃLET ÃTON âĒ KÃķzÃĐpiskolÃĄk, autÃģsiskolÃĄk 9
- 10. MENTSÃNK MEG EGYÃTT ÃLETEKET! KibÃĐdi-Varga Lajos Okl. kÃķzlekedÃĐsmÃĐrnÃķk, PR szakÃĐrtÅ lajos.kibedi@prr.hu 10
