Documentation Management By Sneha
- 1. DOCUMENTATION MANAGEMENT Presented By, Sneha Deshpande
- 2. What is a document? Wikipedia defines aĚýdocumentĚýas, a bounded physical representation of a body ofĚý information Ěýdesigned with the capacity and is usually intended toĚý communicate .
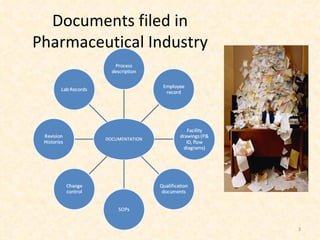
- 3. Documents filed in Pharmaceutical Industry
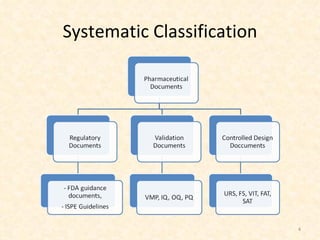
- 5. Some statistics! On an average, for an aseptic process 74 Controlled design documents and 13 Validation Documents (document revisions are not included) are need to prepare, reviewed and approved .
- 6. FDA Guidelines on GDPs GDP’s for Entering Raw data No scratch papers. Use only Labnotebooks Use only pen. No pencils. Sign & date. No backdating is allowed. GDPs on changes made to raw data Sign and date Use of error codes
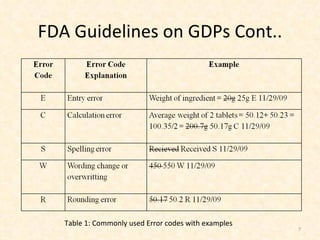
- 7. FDA Guidelines on GDPs Cont.. Table 1: Commonly used Error codes with examples
- 8. FDA Guidelines on GDPs Cont.. General Practices: Follow SOPs Document revision no Correct dating Employee training record
- 9. Trends in Warning letters for not following GDPs Common Observations: Documents were not signed Document Revision number was missing. Document was either not dated or backdated . SOP’s were not followed. Laboratory test records were incomplete. Employee training records were missing. Conclusion: All basic mistakes: sign of mismanagement
- 10. Need for Document management system For paper based systems: 9 +3 =12 Also Makes 20 copies of each document Spends $20 on labor to file each of the document Looses 1 out of 20 office documents Spends $120 on every misfiled document Spends $250 recreating each lost document Spends $25000 to fill four drawer file cabinet and $2000 annually to maintain it
- 11. Document management system (DMS) Document management (DM) can be defined as; creation, storage, organization, transmission, retrieval, manipulation, update, archival and retirement of documents based on organizational needs.
- 12. Document management system (DMS) Cont.. Mathematically; Document Management = (Content Management) + (Attribute Management)
- 13. Electronic Document management System (EDMS) Few such EDMS are enlisted below. ColumbiaSoft 8 OpenDocMan 9 MetricStream 10 M-Files
- 14. Electronic Document management System (EDMS) Basic components of any EDMS: Import tool Tool for storage Indexing tool Distribution tool Security measures
- 15. s Introduced by Motive systems Inc. Type of EDMS Software features: Unique login ID and password Document vault creates a separate drive Check in - check out
- 16. Software features Cont.. Fig: Centralized document storage system offered by M- Files
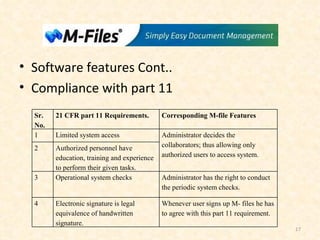
- 17. Software features Cont.. Compliance with part 11 Sr. No. 21 CFR part 11 Requirements. Corresponding M-file Features 1 Limited system access Administrator decides the collaborators; thus allowing only authorized users to access system. 2 Authorized personnel have education, training and experience to perform their given tasks. 3 Operational system checks Administrator has the right to conduct the periodic system checks. 4 Electronic signature is legal equivalence of handwritten signature. Whenever user signs up M- files he has to agree with this part 11 requirement.
- 18. To achieve compliance success: Software system under use is updated documents and procedures are validated Periodic audit trails backup copy of all records
- 19. Conclusion “ If it is not written, it does not exists” Document Management- integral component of Quality management System (QMS) “ Tech-world”- many E-solutions available Use of correct solution.
- 20. References http://www.thefreedictionary.com/document , document, as on 11/29/09 http://en.wikipedia.org/wiki/Document , document, as on 11/29/09 PME 643 lecture 4: Important Project Documents http://www.wcaslab.com/qa/sop/2240v4%20-%20Good%20Documentation%20Practices.pdf , GDPs, as on 11/29/09