1 1 quality-principles
- 1. Principles of quality control of registered medicines, non- registered medicines and counterfeits of medical products Jean-Marc Spieser, Head of Department of Biological Standardisation, OMCL Network & HealthCare (DBO), EDQM/ Council of Europe, Strasbourg
- 2. Content ŌĆó EDQM ŌĆō General Background ŌĆó Legal environment of registered medicines ŌĆó Tools available for the quality control of registered medicines ŌĆó The case of non-registered medicines and their vast environment ŌĆó How to control non-registered products ŌĆó Counterfeits of medical products ŌĆó How, why, and where it happens ŌĆó How to control it Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 2
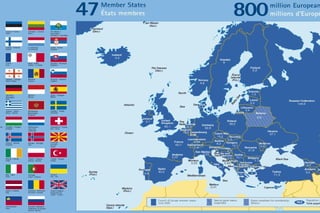
- 3. The Council of Europe ŌĆō Founded in 1949 ŌĆō Development of European common and democratic principles ŌĆō 47 member countries ŌĆō Strasbourg Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 3
- 4. The Council of Europe ŌĆō Founded in 1949 ŌĆō Development of European common and democratic principles ŌĆō 47 member countries ŌĆō Strasbourg European Convention on Human Rights (protection of individuals) & European Court of Human Rights Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 4
- 5. Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 5
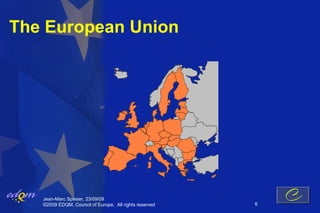
- 6. The European Union Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 6
- 7. EDQM - Short History 1964: ŌĆō Convention on the Elaboration of a European Pharmacopoeia signed by 8 Member States 1992: ŌĆō 1st co-operation contract with the EU Commission on the Biological Standardisation Programme 1994: ŌĆō European Community signs the Convention ŌĆō CEP ŌĆō Implementation of the ŌĆ£Certification of Suitability schemeŌĆØ ŌĆō Official Medicines Control Laboratory (OMCL) ŌĆō Creation of the Network Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 7
- 8. Short History Change of name: the Secretariat of the European Pharmacopoeia becomes the European Department (and later ŌĆ£DirectorateŌĆØ) for the Quality of Medicines (and later ŌĆ£& HealthCareŌĆØ)ŌĆ” Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 8
- 9. Progressive Transfer of Activities 2007 ŌĆō Blood Transfusion and Organ Transplantation 2008 ŌĆō Pharmaceuticals and Pharmaceutical Care (general pharmaceutical activities) 2009 ŌĆō Cosmetics and Food Packaging Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 9
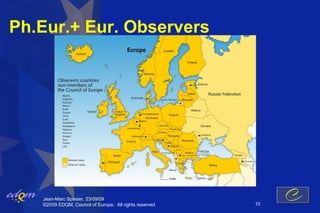
- 10. Ph.Eur.+ Eur. Observers Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 10
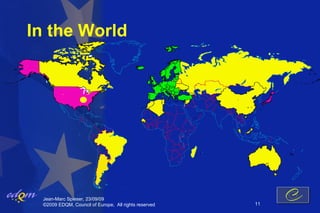
- 11. In the World Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 11
- 12. European Directorate for the Quality of Medicines & HealthCare (EDQM) Mission: to contribute to the basic human right of access to good quality medicines and healthcare Health is a social human right indispensable for the exercise of all other human rights, for prosperity and democratic stability of people in Europe Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 12
- 13. European Directorate for the Quality of Medicines & HealthCare Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 13
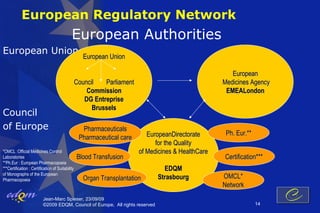
- 14. European Regulatory Network European Authorities European Union European Union European Council Parliament Medicines Agency Commission EMEALondon DG Entreprise Brussels Council of Europe Pharmaceuticals EuropeanDirectorate Ph. Eur.** Pharmaceutical care for the Quality *OMCL :Official Medicines Control of Medicines & HealthCare Laboratories Blood Transfusion Certification*** **Ph.Eur : European Pharmacopoeia ***Certification : Certification of Suitability EDQM of Monographs of the European Pharmacopoeia Organ Transplantation Strasbourg OMCL* Network Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 14
- 15. Quality Assurance Quality is obtained through the combination of: ŌĆó Definition and concept of the product based on development work ŌĆó Good manufacturing processes steadily under control - starting, in-process and final controls - continuous optimisation - VALIDATED controls of the final product based on suitable and appropriate limited testing (necessary and fully sufficient), All the above should be totally traceable Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 15
- 16. Quality Assurance As a general concept QUALITY is manufactured and not only controlled Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 16
- 17. Quality Control QC Principles ŌĆó Laboratory of high quality ’ā╝ Good equipment: validated, regularly maintained ’ā╝ Good reagents ’ā╝ Good operators Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 17
- 18. Quality Control QC Principles ŌĆó Working environment ’ā╝ QA/QMS in place ’ā╝ Good reporting ’ā╝ Procedures ’ā╝ PTS ’ā╝ Follow up and corrective actions ’ā╝ Procedures in place ’ā╝ Training Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 18
- 19. Regulations ŌĆó Medicines are submitted to very strict regulations ŌĆó Each individual preparation requires a Marketing Authorisation (MA) Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 19
- 20. Regulations A fully detailed registration dossier is filed based on: ’é½Quality ŌĆō Production using appropriate process, suitable ingredients controlled and released by validated tests ’é½Safety - toxicology and pharmacology ’é½Efficacy ŌĆō clinical Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 20
- 21. Regulations All information and documentation compiled by producers and/or authorised importer are filed to Health Authorities in accordance with legal procedures and assessed by experts using defined rules ’ā░ Official Authorisation Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 21
- 22. Regulations ŌĆó The product is continually assessed throughout its lifetime: ’āŠ Inspections ’āŠ Controls through laboratory testing ’āŠ Pharmacovigilance If non-conformities are found ’ā░ administrative, legal and/or financial penalities Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 22
- 23. Tools ŌĆó The MAA: QA part ŌĆó CTD part ŌĆó Pharmacopoeias such as National Pharmacopoeias, International Pharmacopoeias, Ph. Eur., USP - General Chapters - Individual Monographs ŌĆó Guidelines/ aide m├®moire for inspections, sampling ŌĆó Literature/ data Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 23
- 24. Sampling ŌĆó Ensure representativity - withdrawn by authority or mandated persons - not by manufacturer - define place where samples are taken, how and how much ŌĆó Ensure good storage - temperature - humidity - transportation Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 24
- 25. Non registered ŌĆ£medicinesŌĆØ ŌĆó Traditional origin - Mostly oral and very restricted data available - Origin not always traceable but should be - How to control the claims and attributes given to these products - What to control, which criteria and indicative parameters ŌĆó Legal environment?? Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 25
- 26. Non registered ŌĆ£medicinesŌĆØ ŌĆó Controls - Based on visual recognition of the substance or the plant - If plant is chopped or ground use of of organoleptic properties, microscopic aspect and basic wet chemistry(test tube ) assays - Important to verify that the right species is there - Need of specialists Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 26
- 27. Non registered ŌĆ£medicinesŌĆØ ŌĆó Controls - Based on traditional knowledge - Sometimes described in literature bust mostly oral - No official standards but need to develop it in known recognised compendia - Be carefull on adulterated, counterfeits - ILLEGAL Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 27
- 28. COUNTERFEITED MEDICINES ŌĆó By definition not known ŌĆó When and where it will happen - ILLEGAL of course by defition - 2 basic areas attacked - Blockbusters, expensive, well known, attractiveŌĆ”ŌĆ”.organised crime - Local , smallsmugglers for small quantities of every possible things Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 28
- 29. COUNTERFEITED MEDICINES ŌĆó How to combat these products - Through multisectorial surveillance - Forensic analysis - Custom vigilance - Police controls Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 29
- 30. COUNTERFEITED MEDICINES ŌĆó To start with ŌĆō Visual check of packaging very important ŌĆó Followed by analysis ŌĆō Simple and more and more sophisticated ŌĆó Need to have comparator ŌĆō Reference substance(s) ŌĆō Authentic samples Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 30
- 31. COUNTERFEITED MEDICINES ŌĆó Simple comparative tests ŌĆó Then sophisticated ŌĆō HPLC using different detectors, coupled with mass spectrum ŌĆó Usefulness of network Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 31
- 32. Thank you! Jean-Marc Spieser, 23/09/09 ┬®2009 EDQM, Council of Europe, All rights reserved 32