5
0 likes163 views
Catalysts speed up chemical reactions by lowering the activation energy of the reaction pathway, without being used up in the process. When added to a reversible reaction, a catalyst increases the rates of both the forward and reverse reactions equally, resulting in no change to the equilibrium position but equilibrium being reached faster. Catalysts are particularly important in industrial applications as they allow reactions to proceed more quickly.
1 of 5
Download to read offline


Ad
Recommended
Activation Energy_final presentation
Activation Energy_final presentationManjot Nijjar
Ã˝
The document discusses activation energy and the Arrhenius equation. It defines activation energy as the minimum amount of energy required for a chemical reaction to occur. The Arrhenius equation relates temperature, activation energy, and the rate constant of a reaction. The document then summarizes an experiment that demonstrates how reaction rate increases with temperature by measuring four reactions at different temperatures and constructing an Arrhenius plot from the results.Reaction rates
Reaction ratesSiyavula
Ã˝
The document discusses reaction rates and factors that affect them, including the nature of reactants, concentration of reactants, temperature, presence of a catalyst, and surface area. It describes collision theory and how it explains how these factors influence reaction rates. Different methods for measuring reaction rates are discussed depending on the type of product produced. The minimum energy required for reactions, known as activation energy, and how temperature and catalysts can provide energy to overcome this are also summarized. Chem 2 - Chemical Kinetics VIII: The Arrhenius Equation, Activation Energy, a...
Chem 2 - Chemical Kinetics VIII: The Arrhenius Equation, Activation Energy, a...Lumen Learning
Ã˝
This document discusses chemical kinetics and the factors that affect reaction rates. It introduces the Arrhenius equation, which relates the rate constant k to temperature, activation energy Ea, and other parameters. Raising the temperature increases the reaction rate by providing more energy to molecules during collisions. Catalysts also increase the reaction rate by lowering the activation energy barrier, changing the reaction mechanism to require less energy. The activation energy is the minimum energy required for molecules to react during collisions. Graphing the Arrhenius equation can determine a reaction's activation energy from experimental data. Catalysts increase the reaction rate without being used up in the reaction or changing its thermodynamics.Energy Changes and Chemical Reactions
Energy Changes and Chemical ReactionsMelinda MacDonald
Ã˝
Chemical reactions require energy to break and form bonds. Exothermic reactions release more energy than they absorb, causing an increase in temperature. Endothermic reactions absorb more energy than they release, causing a decrease in temperature. All reactions require a minimum amount of activation energy to start. The rate of reaction depends on factors like concentration, temperature, surface area, catalysts and inhibitors. Catalysts lower the activation energy and speed up reactions without being used up.Conventional transition state theory
Conventional transition state theoryRahat Inayat Ali
Ã˝
1) Transition state theory (TST) explains reaction rates by assuming a special type of equilibrium between reactants and unstable transition state complexes that have partially formed bonds between reactants and products.
2) TST can be used to calculate activation parameters like enthalpy, entropy and Gibbs energy of activation based on experimentally determined rate constants.
3) According to TST, a reaction will occur if the concentration of the transition state complex is high enough and if the complex breaks apart to form products rather than reverting back to reactants.Factors that affect Reaction Rates
Factors that affect Reaction RatesJm Lucena
Ã˝
Chemical changes occur when substances combine to form new substances and are not reversible without further chemical reactions. The rate of a chemical reaction depends on factors like the nature of reactants, temperature, concentration, surface area, and presence of catalysts. A catalyst lowers the activation energy needed for molecules to collide and react, accelerating the reaction rate without being consumed in the process.Energy Changes
Energy ChangesEmersius
Ã˝
Thermochemistry is the study of heat changes in chemical reactions. Energy is absorbed or released when bonds are broken and formed in a reaction. An exothermic reaction releases heat to the surroundings, while an endothermic reaction absorbs heat from the surroundings. The enthalpy change of a reaction indicates whether it is exothermic or endothermic.Rate of reaction [autosaved]
Rate of reaction [autosaved]damien toh
Ã˝
The document discusses factors that affect the speed of a chemical reaction. It defines reactants as substances that are used up in a reaction and products as substances formed. The speed of a reaction is defined as the rate at which reactants are used up or products formed per unit time. Factors that increase the frequency of effective collisions between reactant particles and thus increase the reaction speed include smaller sizes of solid reactants, presence of catalysts, higher concentrations of reactants in solution, greater pressures of gaseous reactants, and higher temperatures. An example shows that the volume of a gas product increases more quickly at earlier times, indicating a faster reaction speed initially.Collision theory
Collision theorybeckydaw
Ã˝
Collision theory states that for a reaction to occur, particles must collide and the collision must provide enough energy to overcome the activation energy barrier. Reactions with a lower activation energy are more likely to occur. Increasing concentration, temperature, or surface area increases the rate of reaction by providing more opportunities for collisions that can surpass the activation energy. Catalysts also increase reaction rate by lowering the activation energy required.Chemical reactions
Chemical reactionsAllyse Fritz
Ã˝
This document discusses key concepts in chemical reactions and equations. It defines different types of chemical reactions like single displacement, synthesis, and decomposition reactions. It also explains important laws like the law of conservation of energy and mass. It defines exothermic and endothermic reactions as well as concepts like activation energy, catalysts, and inhibitors. Finally, it provides steps for balancing chemical equations.Collision theory and Boltzmann
Collision theory and Boltzmannbeckydaw
Ã˝
Higher temperatures increase the rate of reaction by providing particles with more kinetic energy, leading to more frequent and more energetic collisions that are more likely to exceed the activation energy needed for the reaction to occur. Specifically, for every 10 degree C rise in temperature, the rate of reaction doubles and the time taken halves, as increased particle energy from heat causes more collisions per unit time and a greater chance of particles reaching the activation energy required to react upon colliding.reaction kinetic . collision theory a level
reaction kinetic . collision theory a levelstudying
Ã˝
Only about 1 in 1013 molecular collisions results in a reaction. For a reaction to occur, colliding molecules must have sufficient kinetic energy to overcome the activation energy barrier. Insufficient energy results in the molecules bouncing off each other without reacting. The rate of reaction increases with higher temperature as more molecules then have enough energy to react. A catalyst also increases reaction rate by providing an alternative reaction pathway with lower activation energy. Increasing the concentration of reactants or the surface area of solids increases the number of collisions and reaction rate.Chemical reactions
Chemical reactionstracyconover
Ã˝
This document discusses key concepts in chemistry including types of chemical reactions such as single displacement, synthesis, decomposition, and double displacement reactions. It also covers important laws like the law of conservation of energy and mass. Exothermic and endothermic reactions are defined as well as activation energy, catalysts, and inhibitors. The key components of a balanced chemical equation like reactants and products are outlined. Finally, the six steps for balancing chemical equations are provided.Ch 9.3: Energy Changes and Chemical Reactions
Ch 9.3: Energy Changes and Chemical ReactionsKorrnell Academy: L Class Grade 8 Science
Ã˝
The document discusses energy changes in chemical reactions, particularly in relation to endothermic and exothermic processes, and the concept of activation energy. It outlines factors affecting reaction rates, such as surface area, temperature, concentration, pressure, catalysts, and inhibitors. Additionally, it highlights the role of enzymes as biological catalysts that facilitate essential reactions in living organisms.Chemical reactions
Chemical reactionsAllyse Fritz
Ã˝
This document discusses key concepts in chemistry including types of chemical reactions such as single displacement, synthesis, decomposition, and double displacement reactions. It also covers important laws like conservation of mass and energy. Exothermic and endothermic reactions are defined as well as activation energy, catalysts, and inhibitors. The components of a balanced chemical equation including reactants and products are explained. Finally, the six step process for balancing chemical equations is outlined.Chapter 7 chemical eqilibrium
Chapter 7 chemical eqilibriumNaveed Mallana
Ã˝
This document provides an overview of chemical equilibrium including:
- The law of mass action and how equilibrium is reached through forward and reverse reactions.
- Factors that affect equilibrium position including concentration, pressure, temperature, and catalyst addition based on Le Chatelier's principle.
- Applications of the equilibrium constant including predicting reaction direction and extent.
- Industrial uses of equilibrium concepts such as the Haber process for ammonia synthesis which applies pressure, temperature and product removal.Lect w3 152_d2 - arrhenius and catalysts_alg (1)
Lect w3 152_d2 - arrhenius and catalysts_alg (1)chelss
Ã˝
The document summarizes key concepts about the effects of temperature and catalysts on reaction rates:
1) Increasing temperature generally increases reaction rate because more molecules have enough energy to overcome the activation energy barrier.
2) Catalysts speed up reactions by lowering the activation energy needed, allowing reactions to occur more quickly via a different mechanism.
3) Enzymes are biological catalysts that regulate metabolic reactions in living organisms and act to reduce activation energies.Chem 2 - Chemical Equilibrium X: Le Chatelier's Principle and Temperature Cha...
Chem 2 - Chemical Equilibrium X: Le Chatelier's Principle and Temperature Cha...Lumen Learning
Ã˝
This document discusses how temperature changes act as a stress on chemical equilibriums according to Le Chatelier's principle. It explains that increasing the temperature of an endothermic reaction shifts the equilibrium toward reactants, while decreasing the temperature shifts it toward products. For exothermic reactions, increasing the temperature shifts the equilibrium toward products, while decreasing the temperature shifts it toward reactants. This is because temperature changes are adding or removing heat, which acts as a reactant in endothermic reactions and a product in exothermic reactions.Chapter 18
Chapter 18Malcolm Harrison
Ã˝
1) The rate of a chemical reaction depends on factors like temperature, concentration, particle size, and the use of catalysts. Increasing temperature or concentration generally increases the reaction rate.
2) According to collision theory, particles must collide with sufficient kinetic energy, known as the activation energy, to react. Catalysts lower the activation energy needed for reactions.
3) For reversible reactions, an equilibrium is reached when the rates of the forward and reverse reactions are equal. Equilibrium can be influenced by changing concentrations, temperature, or pressure based on Le Chatelier's principle.2012 topic 6
2012 topic 6David Young
Ã˝
The document summarizes key concepts about rates of reaction from collision theory and kinetic molecular theory. It discusses how the rate of a reaction can be measured by changes in concentration over time and defines instantaneous and average rates. Factors that affect the rate of reaction are concentration, surface area, temperature, and catalysts. Increasing concentration, surface area, or temperature increases the frequency and successful energy of collisions between reactant particles according to collision theory. Catalysts increase the reaction rate by lowering the activation energy needed for reactions.Rate of reaction3
Rate of reaction3Roslinda Rosli
Ã˝
This document discusses factors that affect the rate of chemical reactions according to the collision theory. It explains that for a reaction to occur, reactant particles must collide with sufficient kinetic energy to overcome the activation energy barrier. The rate of reactions depends on both the frequency of collisions between reactants and the activation energy. It then analyzes how increasing the surface area, concentration, temperature, use of catalysts, and pressure can increase the collision frequency, leading to more effective collisions and higher reaction rates.Chemical dynamics, intro,tst, lindemann theory by dr.y. s. thakare
Chemical dynamics, intro,tst, lindemann theory by dr.y. s. thakarepramod padole
Ã˝
This document discusses transition state theory, which was proposed by Henry Eyring, M. Polyani, and M.G. Evans in 1935 to explain chemical reaction rates. It states that for a bimolecular reaction between molecules A2 and B2, the molecules must receive additional energy (activation energy) to form an activated complex/transition state. The activated complex is an unstable high-energy intermediate where the original bonds are partially broken and new bonds between atoms A and B are partially formed. It exists in equilibrium with the reactants. The derivation of the Eyring equation is also presented, which relates the rate of reaction to the thermodynamics of the activated complex. Limitations of the earlier Lindemann theory of unimCellular Energy pt.1
Cellular Energy pt.1Jolie Yu
Ã˝
Cellular processes are driven by exergonic reactions that generate high-energy compounds like ATP. ATP can undergo exergonic reactions to donate phosphate groups or AMP, driving other endergonic reactions like glucose phosphorylation. Cells couple unfavorable reactions to favorable ones through ATP to drive cellular synthesis and transport against gradients. The hydrolysis of ATP and other high-energy compounds provides the energy for these critical energy-requiring cellular processes.Thermodynamics
Thermodynamics–ú–∞–Ω–¥—É—Ö–∞–π –ì.
Ã˝
This document discusses several key concepts in thermodynamics and chemical kinetics:
1. Chemical reactions can proceed partially, producing some products but leaving some original reactants. The rate of reactions is affected by factors like temperature.
2. There are two types of energy - potential energy stored in a system and kinetic energy of motion. Bond formation releases energy while bond breaking absorbs energy.
3. The tendency of reactions to occur spontaneously can be predicted from the free energy change, determined by both entropy change and enthalpy change. Reaction rates depend on factors like activation energy, temperature, concentration, and presence of catalysts.IB Chemistry Collision Theory, Arrhenius Equation and Maxwell Boltzmann Distr...
IB Chemistry Collision Theory, Arrhenius Equation and Maxwell Boltzmann Distr...Lawrence kok
Ã˝
This document provides a tutorial on collision theory, the Arrhenius equation, and the Maxwell-Boltzmann distribution curve. It explains that for a chemical reaction to occur, molecules must collide with the correct orientation and with energy greater than the activation energy. It also discusses how increasing the temperature or concentration of reactants increases the rate of reaction by increasing the frequency and energy of collisions. The Arrhenius equation quantitatively describes the relationship between reaction rate and temperature. The Maxwell-Boltzmann distribution curve illustrates how more molecules have energy above the activation energy at higher temperatures, explaining the temperature dependence of reaction rates.Energy and chemical change
Energy and chemical changeSiyavula
Ã˝
The document discusses energy changes that occur during chemical reactions. It explains that energy is absorbed when bonds in reactants break and released when new bonds in products form. If the energy released is greater than the energy absorbed, the reaction is exothermic and releases heat. If energy absorbed is greater, the reaction is endothermic and absorbs heat. The heat of reaction, ΔH, is calculated as the energy of products minus reactants and is negative for exothermic reactions and positive for endothermic reactions. Activation energy is also defined as the minimum energy needed to start a chemical reaction.Chemical dynamics, intro,collision theory by dr. y. s. thakare
Chemical dynamics, intro,collision theory by dr. y. s. thakarepramod padole
Ã˝
Collision theory proposes that chemical reactions occur when molecules collide with sufficient kinetic energy to overcome the activation energy barrier. The rate of reaction is directly proportional to the number and frequency of effective collisions between reactant molecules possessing energy greater than or equal to the activation energy. However, collision theory has limitations as it does not account for factors like molecular orientation, bond cleavage and formation, or the complexities of reactions involving multi-atomic molecules. It also often overestimates actual reaction rates compared to experimental values.GROUP8_PHYSICALSCIENCE.pptx
GROUP8_PHYSICALSCIENCE.pptxMaryhopeAmancio
Ã˝
The document discusses several key concepts in physical science:
1) Activation energy is needed for chemical reactions to start in order to allow reactants to overcome repulsion forces and break bonds.
2) Enzymes are protein catalysts that accelerate chemical reactions by converting substrates into products.
3) Catalysts lower the transition state energy of a reaction, which in turn lowers the activation energy needed for the reaction.Catalyst and their types
Catalyst and their typesMujeeb UR Rahman
Ã˝
The document discusses catalysts and how they work. It defines a catalyst as a substance that speeds up a chemical reaction but remains unchanged after the reaction. It states that catalysts lower the activation energy of reactions, allowing more effective collisions between reactant molecules. The document distinguishes between homogeneous and heterogeneous catalysts, providing examples of each type. It explains that catalysts can work by initiating reactions through faster intermediate steps or by stabilizing reactive intermediates.Heteropolyacids As Highly Efficient And Green Catalysts Applied In Organic Tr...
Heteropolyacids As Highly Efficient And Green Catalysts Applied In Organic Tr...aulikirw
Ã˝
Heteropolyacids As Highly Efficient And Green Catalysts Applied In Organic Transformations Majid M Heravi
Heteropolyacids As Highly Efficient And Green Catalysts Applied In Organic Transformations Majid M Heravi
Heteropolyacids As Highly Efficient And Green Catalysts Applied In Organic Transformations Majid M HeraviMore Related Content
What's hot (19)
Collision theory
Collision theorybeckydaw
Ã˝
Collision theory states that for a reaction to occur, particles must collide and the collision must provide enough energy to overcome the activation energy barrier. Reactions with a lower activation energy are more likely to occur. Increasing concentration, temperature, or surface area increases the rate of reaction by providing more opportunities for collisions that can surpass the activation energy. Catalysts also increase reaction rate by lowering the activation energy required.Chemical reactions
Chemical reactionsAllyse Fritz
Ã˝
This document discusses key concepts in chemical reactions and equations. It defines different types of chemical reactions like single displacement, synthesis, and decomposition reactions. It also explains important laws like the law of conservation of energy and mass. It defines exothermic and endothermic reactions as well as concepts like activation energy, catalysts, and inhibitors. Finally, it provides steps for balancing chemical equations.Collision theory and Boltzmann
Collision theory and Boltzmannbeckydaw
Ã˝
Higher temperatures increase the rate of reaction by providing particles with more kinetic energy, leading to more frequent and more energetic collisions that are more likely to exceed the activation energy needed for the reaction to occur. Specifically, for every 10 degree C rise in temperature, the rate of reaction doubles and the time taken halves, as increased particle energy from heat causes more collisions per unit time and a greater chance of particles reaching the activation energy required to react upon colliding.reaction kinetic . collision theory a level
reaction kinetic . collision theory a levelstudying
Ã˝
Only about 1 in 1013 molecular collisions results in a reaction. For a reaction to occur, colliding molecules must have sufficient kinetic energy to overcome the activation energy barrier. Insufficient energy results in the molecules bouncing off each other without reacting. The rate of reaction increases with higher temperature as more molecules then have enough energy to react. A catalyst also increases reaction rate by providing an alternative reaction pathway with lower activation energy. Increasing the concentration of reactants or the surface area of solids increases the number of collisions and reaction rate.Chemical reactions
Chemical reactionstracyconover
Ã˝
This document discusses key concepts in chemistry including types of chemical reactions such as single displacement, synthesis, decomposition, and double displacement reactions. It also covers important laws like the law of conservation of energy and mass. Exothermic and endothermic reactions are defined as well as activation energy, catalysts, and inhibitors. The key components of a balanced chemical equation like reactants and products are outlined. Finally, the six steps for balancing chemical equations are provided.Ch 9.3: Energy Changes and Chemical Reactions
Ch 9.3: Energy Changes and Chemical ReactionsKorrnell Academy: L Class Grade 8 Science
Ã˝
The document discusses energy changes in chemical reactions, particularly in relation to endothermic and exothermic processes, and the concept of activation energy. It outlines factors affecting reaction rates, such as surface area, temperature, concentration, pressure, catalysts, and inhibitors. Additionally, it highlights the role of enzymes as biological catalysts that facilitate essential reactions in living organisms.Chemical reactions
Chemical reactionsAllyse Fritz
Ã˝
This document discusses key concepts in chemistry including types of chemical reactions such as single displacement, synthesis, decomposition, and double displacement reactions. It also covers important laws like conservation of mass and energy. Exothermic and endothermic reactions are defined as well as activation energy, catalysts, and inhibitors. The components of a balanced chemical equation including reactants and products are explained. Finally, the six step process for balancing chemical equations is outlined.Chapter 7 chemical eqilibrium
Chapter 7 chemical eqilibriumNaveed Mallana
Ã˝
This document provides an overview of chemical equilibrium including:
- The law of mass action and how equilibrium is reached through forward and reverse reactions.
- Factors that affect equilibrium position including concentration, pressure, temperature, and catalyst addition based on Le Chatelier's principle.
- Applications of the equilibrium constant including predicting reaction direction and extent.
- Industrial uses of equilibrium concepts such as the Haber process for ammonia synthesis which applies pressure, temperature and product removal.Lect w3 152_d2 - arrhenius and catalysts_alg (1)
Lect w3 152_d2 - arrhenius and catalysts_alg (1)chelss
Ã˝
The document summarizes key concepts about the effects of temperature and catalysts on reaction rates:
1) Increasing temperature generally increases reaction rate because more molecules have enough energy to overcome the activation energy barrier.
2) Catalysts speed up reactions by lowering the activation energy needed, allowing reactions to occur more quickly via a different mechanism.
3) Enzymes are biological catalysts that regulate metabolic reactions in living organisms and act to reduce activation energies.Chem 2 - Chemical Equilibrium X: Le Chatelier's Principle and Temperature Cha...
Chem 2 - Chemical Equilibrium X: Le Chatelier's Principle and Temperature Cha...Lumen Learning
Ã˝
This document discusses how temperature changes act as a stress on chemical equilibriums according to Le Chatelier's principle. It explains that increasing the temperature of an endothermic reaction shifts the equilibrium toward reactants, while decreasing the temperature shifts it toward products. For exothermic reactions, increasing the temperature shifts the equilibrium toward products, while decreasing the temperature shifts it toward reactants. This is because temperature changes are adding or removing heat, which acts as a reactant in endothermic reactions and a product in exothermic reactions.Chapter 18
Chapter 18Malcolm Harrison
Ã˝
1) The rate of a chemical reaction depends on factors like temperature, concentration, particle size, and the use of catalysts. Increasing temperature or concentration generally increases the reaction rate.
2) According to collision theory, particles must collide with sufficient kinetic energy, known as the activation energy, to react. Catalysts lower the activation energy needed for reactions.
3) For reversible reactions, an equilibrium is reached when the rates of the forward and reverse reactions are equal. Equilibrium can be influenced by changing concentrations, temperature, or pressure based on Le Chatelier's principle.2012 topic 6
2012 topic 6David Young
Ã˝
The document summarizes key concepts about rates of reaction from collision theory and kinetic molecular theory. It discusses how the rate of a reaction can be measured by changes in concentration over time and defines instantaneous and average rates. Factors that affect the rate of reaction are concentration, surface area, temperature, and catalysts. Increasing concentration, surface area, or temperature increases the frequency and successful energy of collisions between reactant particles according to collision theory. Catalysts increase the reaction rate by lowering the activation energy needed for reactions.Rate of reaction3
Rate of reaction3Roslinda Rosli
Ã˝
This document discusses factors that affect the rate of chemical reactions according to the collision theory. It explains that for a reaction to occur, reactant particles must collide with sufficient kinetic energy to overcome the activation energy barrier. The rate of reactions depends on both the frequency of collisions between reactants and the activation energy. It then analyzes how increasing the surface area, concentration, temperature, use of catalysts, and pressure can increase the collision frequency, leading to more effective collisions and higher reaction rates.Chemical dynamics, intro,tst, lindemann theory by dr.y. s. thakare
Chemical dynamics, intro,tst, lindemann theory by dr.y. s. thakarepramod padole
Ã˝
This document discusses transition state theory, which was proposed by Henry Eyring, M. Polyani, and M.G. Evans in 1935 to explain chemical reaction rates. It states that for a bimolecular reaction between molecules A2 and B2, the molecules must receive additional energy (activation energy) to form an activated complex/transition state. The activated complex is an unstable high-energy intermediate where the original bonds are partially broken and new bonds between atoms A and B are partially formed. It exists in equilibrium with the reactants. The derivation of the Eyring equation is also presented, which relates the rate of reaction to the thermodynamics of the activated complex. Limitations of the earlier Lindemann theory of unimCellular Energy pt.1
Cellular Energy pt.1Jolie Yu
Ã˝
Cellular processes are driven by exergonic reactions that generate high-energy compounds like ATP. ATP can undergo exergonic reactions to donate phosphate groups or AMP, driving other endergonic reactions like glucose phosphorylation. Cells couple unfavorable reactions to favorable ones through ATP to drive cellular synthesis and transport against gradients. The hydrolysis of ATP and other high-energy compounds provides the energy for these critical energy-requiring cellular processes.Thermodynamics
Thermodynamics–ú–∞–Ω–¥—É—Ö–∞–π –ì.
Ã˝
This document discusses several key concepts in thermodynamics and chemical kinetics:
1. Chemical reactions can proceed partially, producing some products but leaving some original reactants. The rate of reactions is affected by factors like temperature.
2. There are two types of energy - potential energy stored in a system and kinetic energy of motion. Bond formation releases energy while bond breaking absorbs energy.
3. The tendency of reactions to occur spontaneously can be predicted from the free energy change, determined by both entropy change and enthalpy change. Reaction rates depend on factors like activation energy, temperature, concentration, and presence of catalysts.IB Chemistry Collision Theory, Arrhenius Equation and Maxwell Boltzmann Distr...
IB Chemistry Collision Theory, Arrhenius Equation and Maxwell Boltzmann Distr...Lawrence kok
Ã˝
This document provides a tutorial on collision theory, the Arrhenius equation, and the Maxwell-Boltzmann distribution curve. It explains that for a chemical reaction to occur, molecules must collide with the correct orientation and with energy greater than the activation energy. It also discusses how increasing the temperature or concentration of reactants increases the rate of reaction by increasing the frequency and energy of collisions. The Arrhenius equation quantitatively describes the relationship between reaction rate and temperature. The Maxwell-Boltzmann distribution curve illustrates how more molecules have energy above the activation energy at higher temperatures, explaining the temperature dependence of reaction rates.Energy and chemical change
Energy and chemical changeSiyavula
Ã˝
The document discusses energy changes that occur during chemical reactions. It explains that energy is absorbed when bonds in reactants break and released when new bonds in products form. If the energy released is greater than the energy absorbed, the reaction is exothermic and releases heat. If energy absorbed is greater, the reaction is endothermic and absorbs heat. The heat of reaction, ΔH, is calculated as the energy of products minus reactants and is negative for exothermic reactions and positive for endothermic reactions. Activation energy is also defined as the minimum energy needed to start a chemical reaction.Chemical dynamics, intro,collision theory by dr. y. s. thakare
Chemical dynamics, intro,collision theory by dr. y. s. thakarepramod padole
Ã˝
Collision theory proposes that chemical reactions occur when molecules collide with sufficient kinetic energy to overcome the activation energy barrier. The rate of reaction is directly proportional to the number and frequency of effective collisions between reactant molecules possessing energy greater than or equal to the activation energy. However, collision theory has limitations as it does not account for factors like molecular orientation, bond cleavage and formation, or the complexities of reactions involving multi-atomic molecules. It also often overestimates actual reaction rates compared to experimental values.Similar to 5 (10)
GROUP8_PHYSICALSCIENCE.pptx
GROUP8_PHYSICALSCIENCE.pptxMaryhopeAmancio
Ã˝
The document discusses several key concepts in physical science:
1) Activation energy is needed for chemical reactions to start in order to allow reactants to overcome repulsion forces and break bonds.
2) Enzymes are protein catalysts that accelerate chemical reactions by converting substrates into products.
3) Catalysts lower the transition state energy of a reaction, which in turn lowers the activation energy needed for the reaction.Catalyst and their types
Catalyst and their typesMujeeb UR Rahman
Ã˝
The document discusses catalysts and how they work. It defines a catalyst as a substance that speeds up a chemical reaction but remains unchanged after the reaction. It states that catalysts lower the activation energy of reactions, allowing more effective collisions between reactant molecules. The document distinguishes between homogeneous and heterogeneous catalysts, providing examples of each type. It explains that catalysts can work by initiating reactions through faster intermediate steps or by stabilizing reactive intermediates.Heteropolyacids As Highly Efficient And Green Catalysts Applied In Organic Tr...
Heteropolyacids As Highly Efficient And Green Catalysts Applied In Organic Tr...aulikirw
Ã˝
Heteropolyacids As Highly Efficient And Green Catalysts Applied In Organic Transformations Majid M Heravi
Heteropolyacids As Highly Efficient And Green Catalysts Applied In Organic Transformations Majid M Heravi
Heteropolyacids As Highly Efficient And Green Catalysts Applied In Organic Transformations Majid M HeraviChapter 18
Chapter 18Malcolm Harrison
Ã˝
The common ion effect occurs when a salt with a common ion is added to a saturated solution of another salt containing that ion. This causes the solubility equilibrium of the original salt to shift left, decreasing the solubility.
When a common ion is added, the concentration of that ion increases on the product side of the solubility equilibrium expression. According to Le Chatelier's principle, this shift in concentration causes the equilibrium to shift left to counteract the change and re-establish equilibrium. Shifting left means more solid salt precipitates out of solution, decreasing the solubility.
So in summary, adding a common ion decreases the solubility of the original salt by shifting its solubility equilibrium left through the common ion effect.Catalysis lecture 2
Catalysis lecture 2Dr. Rabiul Hussain
Ã˝
This document discusses catalytic promoters and inhibitors. It begins by defining promoters as substances added to catalysts that improve activity, selectivity, or stability without being catalysts themselves. Common promoters include molybdenum and aluminum oxide for iron in hydrogen production. Promoters are classified as physical or chemical depending on how they improve catalyst performance. Inhibitors reduce catalyst activity and are useful for controlling side reactions. The document also discusses mechanisms of promotion and inhibition, as well as autocatalysis and catalytic poisoning. It explains how catalysts lower the activation energy of reactions, providing an alternative reaction pathway and increasing reaction rates.Catalysts Notes
Catalysts Notesericchapman81
Ã˝
Cells use catalysts to speed up chemical reactions by reducing the activation energy required. Catalysts work by lowering the energy barrier of a reaction without being used up in the process, allowing reactions to occur faster and with less energy. The most common type of catalyst in living things are enzymes, which are proteins that catalyze biochemical reactions and help processes like digestion occur quicker without changing their own structure.Catalyst and their types
Catalyst and their typesMujeeb UR Rahman
Ã˝
A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being consumed in the process. Common types of catalysts include enzymes, acid-base catalysts, and heterogeneous or surface catalysts. Catalysts work by either lowering the transition state energy of a reaction, thereby reducing the activation energy, or by changing the reaction mechanism.Rate and extent of reaction
Rate and extent of reactionSiyavula
Ã˝
This document discusses reaction rates and factors that affect reaction rates. It explains that reaction rate is calculated based on the moles of product formed or reactants used over the reaction time. The rate of reaction is affected by properties of the reactants, concentration, surface area, pressure, temperature, and presence of a catalyst. Various methods can be used to measure reaction rates depending on the products, such as collecting gases, measuring precipitates, or observing color changes. For a reaction to occur, particles must collide with sufficient energy to overcome the activation energy, and increasing temperature or adding a catalyst can provide this energy. A catalyst lowers the activation energy and speeds up the reaction but remains unchanged after.chapter_16.ppt
chapter_16.pptGlaiza Valdez-Abucay
Ã˝
This document discusses factors that affect the rate of chemical reactions and key concepts related to reaction rates. It covers how reaction rate depends on concentration, temperature, surface area, and catalysts. Catalysts lower the activation energy of a reaction, increasing the rate. Reaction rate can be determined from measurements of reactant disappearance or product appearance over time. The rate law equation describes the quantitative relationship between reaction rate and concentrations.B.1 Collision Theory PHYSICAL SCIENCE.pptx
B.1 Collision Theory PHYSICAL SCIENCE.pptxMAHAZELTEOLOGO3
Ã˝
The document discusses collision theory, which explains that for a chemical reaction to occur, reactant particles must collide with sufficient energy to overcome the activation energy barrier. It explains how factors like concentration, temperature, surface area, and catalysts affect the rate of reaction by influencing the frequency and success of collisions between reactants. Catalysts specifically provide an alternative reaction pathway with lower activation energy.Ad
More from shaunoff (20)
5. using electromagnets
5. using electromagnetsshaunoff
Ã˝
Lifts, cars, and other large electrical machines use high currents. Relays use an electromagnet to allow a small current in one circuit to control a large current in another circuit. This allows a small current to control whether a large current flows through another circuit by closing or opening a switch.2
2shaunoff
Ã˝
The circuit for a door bell contains an electromagnet that pulls an armature towards it when the circuit is closed, causing a hammer to strike the bell and produce sound. The movement of the armature then breaks the circuit, allowing the hammer to return to its original position, and this sequence repeats to make the bell ring continuously whenever the door bell button is pressed.1
1shaunoff
Ã˝
An electromagnet on a recycling plant conveyor belt is used to pick up and move metal cans. Electromagnets attract ferromagnetic metals like iron, nickel, and cobalt. Electromagnets have the advantage over permanent magnets in that their magnetic field can be switched on and off by controlling the electric current running through the magnet coils.5
5shaunoff
Ã˝
The document presents results from an experiment investigating electromagnets. It shows that increasing the number of coils or the current in an electromagnet increases the number of drawing pins attracted. Graphs illustrate the direct relationship between the number of coils/current and the number of drawing pins attracted.4
4shaunoff
Ã˝
An experiment was designed to investigate how changing the current and number of coils affects the strength of an electromagnet. The apparatus shown could be used by altering the current running through the coils or changing the number of coils wrapped around the iron core, then measuring the effect on the magnetic force produced. Results could show that increasing either the current or number of coils strengthens the electromagnet.3
3shaunoff
Ã˝
The document discusses two experiments that can investigate factors affecting the strength of an electromagnet other than the presence of an iron core. The first experiment would examine how the number of coils influences the number of drawing pins attracted while keeping current the same. The second would look at how the size of the current affects the number of pins for a constant number of coils.6. halides
6. halidesshaunoff
Ã˝
Halides are compounds formed between halogens (F, Cl, Br, I) and metals. They have many industrial, medical, and household applications. Halides can be identified by their reaction with silver nitrate solution to form precipitates of insoluble silver halides. The hydrogen halides are colorless gases with hydrogen fluoride having an unexpectedly high boiling point due to hydrogen bonding between H-F molecules. Larger halide ions are more reactive reducing agents as their outer electrons are farther from the nucleus and more easily donated.4. g7 and g1
4. g7 and g1shaunoff
Ã˝
Alkali metals react vigorously with chlorine to form metal chlorides. Lithium reacts with chlorine gas to form lithium chloride according to the chemical equation 2Li (s) + Cl2 (g) ‚Üí 2LiCl (s). Sodium also reacts with chlorine gas in a similar reaction to form sodium chloride, represented by the equation 2Na (s) + Cl2 (g) ‚Üí 2NaCl (s).3. group 7
3. group 7shaunoff
Ã˝
The document discusses the chemical properties of the halogens (fluorine, chlorine, bromine, iodine, astatine) by examining their reactivity trends down the group in reactions with iron wool and hydrogen. It notes that reactivity decreases down the group, with chlorine causing iron wool to burn brightly, bromine less so, and iodine only slightly. It also examines the halogens' reactions with metals like sodium and nonmetals like hydrogen to form halides, and displacement reactions between halogens and halide ions.2. group 1
2. group 1shaunoff
Ã˝
The document discusses the chemical properties of alkali metals. It explains that alkali metals react vigorously with oxygen and water. The reactivity increases down the group as the atoms get larger, shielding the outer electrons from the nucleus and making them easier to lose. Equations for reactions of lithium, sodium, and potassium with oxygen, water, and other substances are provided. Flame tests for group 2 metals are also discussed.5.displacement
5.displacementshaunoff
Ã˝
The document discusses the chemical properties of halogens, specifically their oxidizing abilities in displacement reactions. It states that in displacement reactions between halogens and halides, the halogen acts as an oxidizing agent by oxidizing the halide ion and being reduced to form the halide ion itself. The order of increasing oxidizing ability among the halogens is F > Cl > Br > I. An example reaction of chlorine displacing bromine is provided, and it is explained that in the reaction, chlorine is reduced while bromide is oxidized.1. group 0
1. group 0shaunoff
Ã˝
The document discusses the properties and discovery of noble gases. It notes that:
- Noble gases (group 0) are very unreactive due to their full outer electron shells. This stability makes them similar across the group.
- Helium was first discovered on the sun in 1868. Argon was discovered in the 1890s after nitrogen from air was found to contain a small quantity of an inert element.
- Ramsay realized argon needed its own group and later discovered it contained traces of other noble gases - neon, krypton, and xenon. Radon was discovered in 1900.4
4shaunoff
Ã˝
The document discusses how changing the pressure affects the equilibrium of reversible gas reactions. It states that if the pressure is increased, the equilibrium will shift in the direction that decreases the number of gas molecules. If the pressure is decreased, the equilibrium will shift in the direction that increases the number of gas molecules. It also provides the example of nitrogen dioxide and dinitrogen tetroxide, explaining that if the pressure of this system is increased, more dinitrogen tetroxide will be produced.3
3shaunoff
Ã˝
Increasing the concentration of a substance in a reversible reaction shifts the equilibrium to decrease the concentration of that substance. For example, adding more water to a reaction producing bismuth oxychloride and hydrochloric acid from bismuth chloride and water would shift the equilibrium right, producing more products. Similarly, adding more chlorine gas to a reaction producing iodine trichloride from chlorine and iodine chloride would make the mixture appear more yellow by shifting the equilibrium to the right.2
2shaunoff
Ã˝
The document discusses exothermic and endothermic reactions. It states that all reactions are exothermic in one direction, releasing heat, and endothermic in the other direction, absorbing heat. It also explains that if temperature is increased, the equilibrium will shift in the endothermic direction to decrease temperature, and if temperature is decreased, the equilibrium will shift in the exothermic direction to increase temperature. It provides the example of nitrogen dioxide and dinitrogen tetroxide, where the forward reaction is exothermic and the backward reaction is endothermic. If temperature is increased in this system, more nitrogen dioxide will be produced.6
6shaunoff
Ã˝
Ammonia is produced industrially through the Haber process, which involves reacting nitrogen and hydrogen at high pressures and temperatures. However, the reaction is reversible and does not go to completion. To maximize yield while keeping costs low, manufacturers use a compromise of 450°C and 200 atmospheres, as extremely high pressures require expensive equipment and low temperatures slow the reaction rate. Key costs in ammonia production include raw materials, energy, equipment, wages. Yield is maximized by using a catalyst, removing ammonia as it forms, and recycling unreacted gases.1
1shaunoff
Ã˝
Le Chatelier's principle states that if a system in dynamic equilibrium experiences a change, the equilibrium will shift to counteract the change. Specifically, increasing temperature shifts equilibrium towards the endothermic direction, increasing concentration shifts equilibrium away from that substance, and increasing pressure shifts equilibrium away from the gas producing side.IB 3.2.2.5
IB 3.2.2.5shaunoff
Ã˝
The melting points of elements in group 1 decrease down the group because the atomic radius increases, resulting in weaker metallic bonds and a weaker attraction between the nucleus and delocalized electrons. The melting points given for lithium, sodium, potassium, rubidium, and cesium show this decreasing trend.IB 3.2.2.4
IB 3.2.2.4shaunoff
Ã˝
Electronegativity decreases down a group because the atomic radius increases and shielding increases due to electrons filling new principal energy levels, making the increased nuclear charge less significant. Therefore, iodine has a larger atomic radius and attracts electron density in a covalent bond less strongly than fluorine.IB3.2.2.2
IB3.2.2.2shaunoff
Ã˝
The document discusses trends in ionic radii for cations and anions. It explains that for both group 1 and group 7 elements, ionic radii increase down the group as the outer energy level moves further from the nucleus. For cations, ionic radii are smaller than atomic radii because cations have fewer electrons and greater nuclear attraction. For anions, ionic radii are larger than atomic radii because anions have more electrons and weaker nuclear attraction. Tables of atomic and ionic radii are provided for alkali metals and halogens to illustrate these trends.Ad
5
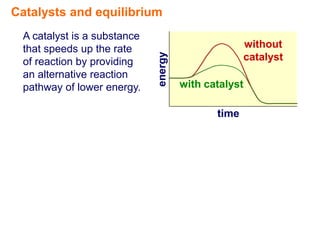
- 2. Catalysts and equilibrium A catalyst is a substance that speeds up the rate of reaction by providing an alternative reaction pathway of lower energy.
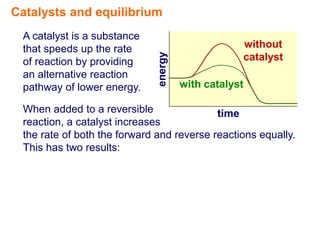
- 3. Catalysts and equilibrium A catalyst is a substance that speeds up the rate without energy of reaction by providing catalyst an alternative reaction pathway of lower energy. with catalyst time
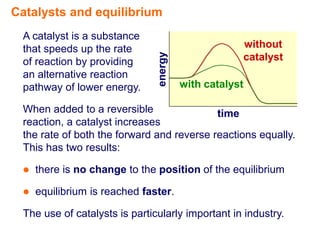
- 4. Catalysts and equilibrium A catalyst is a substance that speeds up the rate without energy of reaction by providing catalyst an alternative reaction pathway of lower energy. with catalyst When added to a reversible time reaction, a catalyst increases the rate of both the forward and reverse reactions equally. This has two results:
- 5. Catalysts and equilibrium A catalyst is a substance that speeds up the rate without energy of reaction by providing catalyst an alternative reaction pathway of lower energy. with catalyst When added to a reversible time reaction, a catalyst increases the rate of both the forward and reverse reactions equally. This has two results:  there is no change to the position of the equilibrium  equilibrium is reached faster. The use of catalysts is particularly important in industry.