Atomic history day #2 1
- 1. Ernest Rutherford (1871-1937) ÔÇó Learned physics in J.J. ThomsonÔÇÖ lab. ÔÇó Noticed that ÔÇÿalphaÔÇÖ particles were sometime deflected by something in the air. ÔÇó Gold-foil experiment
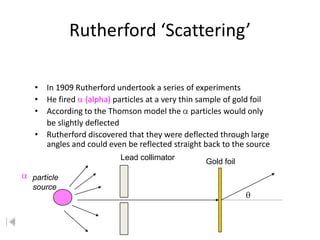
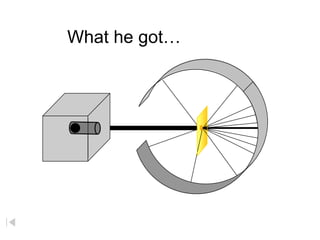
- 2. Rutherford ÔÇÿScatteringÔÇÖ ÔÇó In 1909 Rutherford undertook a series of experiments ÔÇó He fired (alpha) particles at a very thin sample of gold foil ÔÇó According to the Thomson model the particles would only be slightly deflected ÔÇó Rutherford discovered that they were deflected through large angles and could even be reflected straight back to the source Lead collimator Gold foil particle source
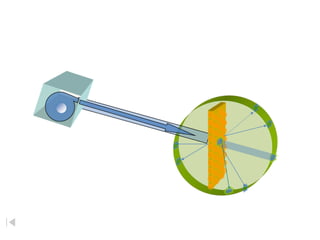
- 3. RutherfordÔÇÖs Apparatus beam of alpha particles radioactive substance fluorescent screen circular - ZnS coated gold foil Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 120
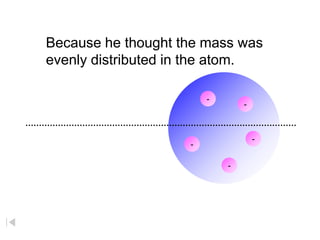
- 5. Because he thought the mass was evenly distributed in the atom. - - - - -
- 6. Because, he thought the mass was evenly - - distributed in the atom - - - - - - - - - - - - - - - - - - - - - - -
- 8. Density and the Atom ÔÇó Since most of the particles went through, the atom was mostly empty. ÔÇó Because the alpha rays were deflected so much, the positive pieces it was striking were heavy. ÔÇó Small volume and big mass = big density ÔÇó This small dense positive area is the nucleus California WEB
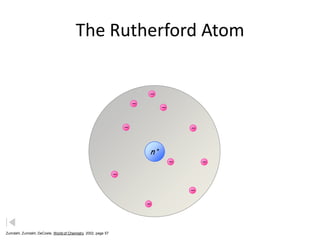
- 9. The Rutherford Atom n+ Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 57
- 10. Niels Bohr ÔÇó In the Bohr Model (1913) the neutrons and protons occupy a dense central region called the nucleus, and the electrons orbit the nucleus much like planets orbiting the Sun. ÔÇó They are not confined to a planar orbit like the planets are.
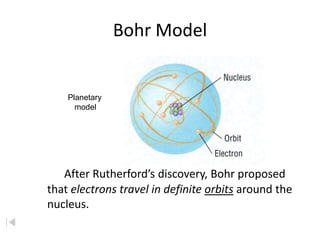
- 11. Bohr Model Planetary model After RutherfordÔÇÖs discovery, Bohr proposed that electrons travel in definite orbits around the nucleus.