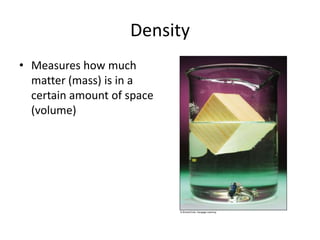
Density
Download as PPTX, PDF0 likes425 views
This document discusses density and how to calculate it by dividing mass by volume. It provides the formula, D = m/v, and examples of calculating density when given the mass and volume of different objects, or using density and one value to find the other. Density can be used to determine if an object will float or sink in water based on its density compared to the density of water.
1 of 6
Downloaded 21 times





Recommended
Biology density practice problems 



Biology density practice problems M, Michelle Jeannite
Ã˝
This document contains 16 practice problems about calculating density, volume, and mass using given values. The problems involve objects like platinum bars, lead cylinders, cork, glass bottles, soap bars, metal sheets, pencils, water, wood blocks, rocks, boulders, and gold nuggets. Learners are asked to use density, volume, and mass formulas to solve for unknown values in each problem.What is the different between the net pay and resrvoir thickness



What is the different between the net pay and resrvoir thicknessStudent
Ã˝
Prepared by Yasir Albeatiy
Contact me with information below:
E-Mail: yasiralbeatiy2015@gmail.com
Phone No. + Whatsapp : +9647828319225
Facebook Page: www.facebook.com/petroleumengineeringz
Calculating And Finding Density



Calculating And Finding DensityBrian Cunningham
Ã˝
To calculate density, students will find the mass of various objects using a triple beam balance and determine their volumes using the water displacement method with a graduated cylinder. Density is calculated by dividing an object's mass by its volume (D=m/v). Students will first find the mass and empty volume of a vial, then use water displacement to determine the volume of additional objects by subtracting the initial water level from the level with the object submerged.Applications of trignometry



Applications of trignometryAbhijit Jadhav
Ã˝
Trigonometry is a branch of mathematics used to define relationships between sides and angles of triangles, especially right triangles. It has applications in fields like architecture, astronomy, geology, navigation, and oceanography. Trigonometric functions like sine, cosine, and tangent are ratios that relate sides and angles, and trigonometry allows distances, heights, and depths to be easily calculated. Architects use trigonometry to design buildings, astronomers use it to measure distances to stars, and geologists use it to determine slope stability.Thermo#2



Thermo#2gbsliebs2002
Ã˝
This document defines different types of systems and their interactions with surroundings in thermodynamics. An open system can exchange both matter and energy with surroundings, a closed system can only exchange energy, and an isolated system cannot exchange either. Exothermic processes release heat to surroundings while endothermic processes absorb heat from surroundings. Vaporization and melting of substances are endothermic since they require energy, while freezing is exothermic as it releases energy.Thermo#1



Thermo#1gbsliebs2002
Ã˝
Total energy in the universe is constant and can be transformed between kinetic and potential energy. Kinetic energy is the energy of motion, while potential energy is stored energy due to an object's position. The specific heat capacity of a substance relates the amount of heat absorbed or released to the temperature change, and allows the calculation of heat transfer using the formula: heat (q) = mass x specific heat x temperature change (ΔT).Gases pt.1



Gases pt.1gbsliebs2002
Ã˝
Pressure is force per unit area measured in pounds per square inch (psi) or pascals (Pa). Dalton's law of partial pressures states that the total pressure of a gas mixture equals the sum of the individual gas partial pressures. Boyle's law describes the inverse relationship between the pressure and volume of a gas at a constant temperature. Charles' law states that the volume of a gas increases or decreases proportionally with an increase or decrease in temperature when pressure is kept constant.Stoichiometric calculations



Stoichiometric calculationsgbsliebs2002
Ã˝
This document discusses stoichiometric calculations and concepts like limiting reactants, theoretical yield, and percent yield. It explains that the coefficients in a balanced chemical equation give the ratio of moles of reactants and products. It also describes how to use the molar ratios to calculate the mass of a product formed or reactant used from the mass of another substance. Sample stoichiometry problems are provided that involve calculating moles or masses of substances. The concepts of limiting reactant, theoretical yield, and percent yield are defined.Balancing equations



Balancing equationsgbsliebs2002
Ã˝
1. This document discusses different types of chemical reactions including synthesis, decomposition, single replacement, double replacement, and combustion reactions. It provides examples of each type and explains the general formulas and patterns involved.
2. Synthesis reactions involve two or more reactants combining to form a single product. Decomposition reactions involve a single reactant breaking down into simpler products. Replacement reactions involve an element replacing another in a compound.
3. Combustion reactions involve hydrocarbons reacting with oxygen to produce carbon dioxide and water, and are important in processes like burning fuels and respiration.Periodic table



Periodic tablegbsliebs2002
Ã˝
Dmitri Mendeleev arranged the elements in the periodic table based on their chemical properties and left spaces for elements that were yet to be discovered. He was able to predict properties of these unknown elements accurately, including scandium, gallium, and germanium, which were later discovered with properties matching his predictions.Atomic strucutre



Atomic strucutregbsliebs2002
Ã˝
This document discusses the basic properties of protons, neutrons, electrons, and isotopes. Protons have a positive charge and determine atomic number. Neutrons have no charge and equal mass to protons. Electrons have a negative charge and negligible mass. Isotopes are atoms of the same element with different numbers of neutrons, resulting in the same atomic number but different mass numbers.Atomic history day #2 1



Atomic history day #2 1gbsliebs2002
Ã˝
Ernest Rutherford discovered the structure of the atom through his gold foil experiment in 1909. He fired alpha particles at a thin gold foil and discovered that some particles were deflected at very large angles or reflected straight back, contrary to existing models of the atom. This led him to conclude that atoms have a very small, dense, positively charged nucleus at their center, with electrons orbiting the nucleus. Niels Bohr later expanded on this model by proposing that electrons travel in definite orbits around the nucleus.Atomic history day #2 1



Atomic history day #2 1gbsliebs2002
Ã˝
Ernest Rutherford discovered the structure of the atom through his gold foil experiment in 1909. He fired alpha particles at a thin gold foil and discovered that some particles were deflected at very large angles or reflected straight back, contrary to expectations. This showed that the mass of an atom is concentrated in a very small, dense central nucleus. Niels Bohr then built on this work by proposing in 1913 that electrons orbit the nucleus in fixed orbits, establishing the early nuclear model of the atom.Atomic history day #1



Atomic history day #1gbsliebs2002
Ã˝
Democritus and Leucippus were ancient Greek philosophers who first proposed the idea of atoms in the 5th century BC. They believed that all matter was made of extremely small, indivisible particles called atoms. This was the first atomic theory, though it was not supported by experiments. Later, in the late 1700s, John Dalton revived the atomic theory with experimental evidence and proposed three main laws of atomic theory: the law of conservation of mass, the law of definite proportions, and the law of multiple proportions. Dalton proposed that atoms of different elements have different masses and that compounds are formed by atoms of different elements combining.Measurements and sig figs



Measurements and sig figsgbsliebs2002
Ã˝
Precision refers to the reproducibility of measurements while accuracy refers to how close a measurement is to the true value. All measurements must include units and use the metric system of grams, meters, and liters. Temperature is measured in Celsius with the formula to convert to Fahrenheit. When taking measurements, always estimate one digit past the last readable digit to show uncertainty. Significant figures indicate the number of digits that are certain in a measurement based on rules like nonzero integers and captive zeros counting as significant. Mathematical operations follow rules where addition and subtraction take the least precise measurement's decimal places as significant figures and multiplication and division take the least precise measurement's significant figures. Scientific notation allows easy conversion between decimal and exponential forms while maintaining the same value.Physical properties



Physical propertiesgbsliebs2002
Ã˝
Physical properties describe characteristics like color, shape, and texture that do not change a substance's identity. Physical changes alter these properties through melting, boiling, or smashing without forming a new substance, while chemical properties and changes involve atomic and molecular transformations that create different materials.Representing matter



Representing mattergbsliebs2002
Ã˝
The fundamental unit of matter is the atom. Elements contain only one type of atom and have distinct physical and chemical properties, while compounds contain two or more different types of atoms bonded together. Pure substances have a fixed composition and cannot be separated into simpler substances through physical changes alone. Mixtures have a variable composition and their components can be separated into pure substances using physical methods.The concept of equilibrium



The concept of equilibriumgbsliebs2002
Ã˝
The document discusses chemical equilibrium, which occurs when the forward and reverse reactions of a chemical reaction proceed at the same rate. At equilibrium, the concentrations of reactants and products remain constant. The equilibrium constant, K, is a ratio of products over reactants that characterizes the position of equilibrium. A large K value indicates the reaction favors products, while a small K value indicates the reaction favors reactants. Le châtelier’s principle



Le châtelier’s principlegbsliebs2002
Ã˝
Le Ch√¢telier's Principle states that if a stress is applied to a system at equilibrium, the system will adjust to partially counteract the stress and reach a new equilibrium position. Changes in concentration, pressure, volume, or temperature can act as stresses. For example, increasing the concentration of reactants will shift the equilibrium to the product side. A catalyst will speed the rate of both the forward and reverse reactions but will not change the equilibrium position or constant.Collison Theory



Collison Theorygbsliebs2002
Ã˝
Collisions between reactants are necessary for chemical reactions to occur. The reactants must collide with the proper orientation and with sufficient kinetic energy to overcome the activation energy barrier. Adding a catalyst can also increase the reaction rate by lowering the activation energy needed for collisions between reactants to be productive.Atomic Structure



Atomic Structuregbsliebs2002
Ã˝
The document discusses the wave-particle duality of light and electrons. It describes how Max Planck proposed that electromagnetic radiation was quantized into discrete packets of energy called quanta or photons. Later, Albert Einstein applied this idea and suggested that light consists of quantized packets or photons. The document also discusses how Louis de Broglie applied wave-particle duality to electrons in 1924, showing that electrons can exhibit wave properties. This led to the development of quantum mechanics and the modern quantum mechanical model of the atom, which uses orbitals and quantum numbers to describe where electrons are likely to be found.Thermo#2



Thermo#2gbsliebs2002
Ã˝
This document defines different types of systems and their interactions with surroundings in thermodynamics. An open system can exchange both matter and energy with surroundings, a closed system can only exchange energy, and an isolated system cannot exchange either. Exothermic processes release heat to surroundings while endothermic processes absorb heat from surroundings. Specific examples of endothermic processes that require energy input include vaporization and melting, while freezing is exothermic and releases energy.Thermo#1



Thermo#1gbsliebs2002
Ã˝
Energy exists in two main forms - kinetic energy, which is energy due to motion, and potential energy, which is stored energy due to position. The total energy of a system is the sum of its kinetic and potential energies, and the total energy of the universe remains constant according to the law of conservation of energy. Heat is a transfer of energy between objects due to a temperature difference, while temperature measures the average kinetic energy of particles. The amount of heat required to change an object's temperature depends on its heat capacity, which relates the heat supplied to the temperature change.Gases Pt.1



Gases Pt.1gbsliebs2002
Ã˝
Pressure is force per unit area measured in pounds per square inch (psi) or pascals (Pa). Dalton's law of partial pressures states that the total pressure of a gas mixture equals the sum of the individual gas partial pressures. Boyle's law describes the inverse relationship between the pressure and volume of a gas at a constant temperature. Charles' law states that the volume of a gas increases or decreases proportionally with an increase or decrease in temperature when pressure remains constant.Stoichiometric Calculations



Stoichiometric Calculationsgbsliebs2002
Ã˝
The document discusses stoichiometric calculations and limiting reactants. It explains that stoichiometric calculations use the mole ratios from balanced chemical equations to determine the amount of reactants and products. The limiting reactant is the one that limits the amount of product that can be formed. The theoretical yield is the maximum amount of product possible based on the stoichiometry, while the actual yield is the amount experimentally obtained. Percent yield compares the actual and theoretical yields.Balancing Equations



Balancing Equationsgbsliebs2002
Ã˝
This document provides information on different types of chemical reactions:
1) Balancing equations must follow the law of conservation of mass and changing coefficients to balance atoms. Hydrogen and oxygen should be balanced last.
2) Synthesis reactions combine reactants to form a product. Decomposition reactions break compounds into simpler substances. Single replacement reactions involve one element replacing another in a compound.
3) Double replacement reactions involve the exchange of ions between reactants to form new ionic compounds as products. Products are determined by pairing ions that are "across" from each other.Moles



Molesgbsliebs2002
Ã˝
The document discusses moles, which are a unit used to measure very large quantities in chemistry. A mole is defined as 6.02 x 1023 particles, such as atoms, molecules, ions. This number is known as Avogadro's number. The document provides examples of how many everyday items would equal a mole to demonstrate just how large a mole is. It also discusses how moles can be used to convert between the number of particles, moles, and mass in grams.Periodic Table



Periodic Tablegbsliebs2002
Ã˝
Dmitri Mendeleev arranged the elements in the periodic table based on their chemical properties and left spaces for elements that were yet to be discovered. He was able to predict properties of these unknown elements accurately, including scandium, gallium, and germanium, which were later discovered with properties matching his predictions.More Related Content
More from gbsliebs2002 (20)
Balancing equations



Balancing equationsgbsliebs2002
Ã˝
1. This document discusses different types of chemical reactions including synthesis, decomposition, single replacement, double replacement, and combustion reactions. It provides examples of each type and explains the general formulas and patterns involved.
2. Synthesis reactions involve two or more reactants combining to form a single product. Decomposition reactions involve a single reactant breaking down into simpler products. Replacement reactions involve an element replacing another in a compound.
3. Combustion reactions involve hydrocarbons reacting with oxygen to produce carbon dioxide and water, and are important in processes like burning fuels and respiration.Periodic table



Periodic tablegbsliebs2002
Ã˝
Dmitri Mendeleev arranged the elements in the periodic table based on their chemical properties and left spaces for elements that were yet to be discovered. He was able to predict properties of these unknown elements accurately, including scandium, gallium, and germanium, which were later discovered with properties matching his predictions.Atomic strucutre



Atomic strucutregbsliebs2002
Ã˝
This document discusses the basic properties of protons, neutrons, electrons, and isotopes. Protons have a positive charge and determine atomic number. Neutrons have no charge and equal mass to protons. Electrons have a negative charge and negligible mass. Isotopes are atoms of the same element with different numbers of neutrons, resulting in the same atomic number but different mass numbers.Atomic history day #2 1



Atomic history day #2 1gbsliebs2002
Ã˝
Ernest Rutherford discovered the structure of the atom through his gold foil experiment in 1909. He fired alpha particles at a thin gold foil and discovered that some particles were deflected at very large angles or reflected straight back, contrary to existing models of the atom. This led him to conclude that atoms have a very small, dense, positively charged nucleus at their center, with electrons orbiting the nucleus. Niels Bohr later expanded on this model by proposing that electrons travel in definite orbits around the nucleus.Atomic history day #2 1



Atomic history day #2 1gbsliebs2002
Ã˝
Ernest Rutherford discovered the structure of the atom through his gold foil experiment in 1909. He fired alpha particles at a thin gold foil and discovered that some particles were deflected at very large angles or reflected straight back, contrary to expectations. This showed that the mass of an atom is concentrated in a very small, dense central nucleus. Niels Bohr then built on this work by proposing in 1913 that electrons orbit the nucleus in fixed orbits, establishing the early nuclear model of the atom.Atomic history day #1



Atomic history day #1gbsliebs2002
Ã˝
Democritus and Leucippus were ancient Greek philosophers who first proposed the idea of atoms in the 5th century BC. They believed that all matter was made of extremely small, indivisible particles called atoms. This was the first atomic theory, though it was not supported by experiments. Later, in the late 1700s, John Dalton revived the atomic theory with experimental evidence and proposed three main laws of atomic theory: the law of conservation of mass, the law of definite proportions, and the law of multiple proportions. Dalton proposed that atoms of different elements have different masses and that compounds are formed by atoms of different elements combining.Measurements and sig figs



Measurements and sig figsgbsliebs2002
Ã˝
Precision refers to the reproducibility of measurements while accuracy refers to how close a measurement is to the true value. All measurements must include units and use the metric system of grams, meters, and liters. Temperature is measured in Celsius with the formula to convert to Fahrenheit. When taking measurements, always estimate one digit past the last readable digit to show uncertainty. Significant figures indicate the number of digits that are certain in a measurement based on rules like nonzero integers and captive zeros counting as significant. Mathematical operations follow rules where addition and subtraction take the least precise measurement's decimal places as significant figures and multiplication and division take the least precise measurement's significant figures. Scientific notation allows easy conversion between decimal and exponential forms while maintaining the same value.Physical properties



Physical propertiesgbsliebs2002
Ã˝
Physical properties describe characteristics like color, shape, and texture that do not change a substance's identity. Physical changes alter these properties through melting, boiling, or smashing without forming a new substance, while chemical properties and changes involve atomic and molecular transformations that create different materials.Representing matter



Representing mattergbsliebs2002
Ã˝
The fundamental unit of matter is the atom. Elements contain only one type of atom and have distinct physical and chemical properties, while compounds contain two or more different types of atoms bonded together. Pure substances have a fixed composition and cannot be separated into simpler substances through physical changes alone. Mixtures have a variable composition and their components can be separated into pure substances using physical methods.The concept of equilibrium



The concept of equilibriumgbsliebs2002
Ã˝
The document discusses chemical equilibrium, which occurs when the forward and reverse reactions of a chemical reaction proceed at the same rate. At equilibrium, the concentrations of reactants and products remain constant. The equilibrium constant, K, is a ratio of products over reactants that characterizes the position of equilibrium. A large K value indicates the reaction favors products, while a small K value indicates the reaction favors reactants. Le châtelier’s principle



Le châtelier’s principlegbsliebs2002
Ã˝
Le Ch√¢telier's Principle states that if a stress is applied to a system at equilibrium, the system will adjust to partially counteract the stress and reach a new equilibrium position. Changes in concentration, pressure, volume, or temperature can act as stresses. For example, increasing the concentration of reactants will shift the equilibrium to the product side. A catalyst will speed the rate of both the forward and reverse reactions but will not change the equilibrium position or constant.Collison Theory



Collison Theorygbsliebs2002
Ã˝
Collisions between reactants are necessary for chemical reactions to occur. The reactants must collide with the proper orientation and with sufficient kinetic energy to overcome the activation energy barrier. Adding a catalyst can also increase the reaction rate by lowering the activation energy needed for collisions between reactants to be productive.Atomic Structure



Atomic Structuregbsliebs2002
Ã˝
The document discusses the wave-particle duality of light and electrons. It describes how Max Planck proposed that electromagnetic radiation was quantized into discrete packets of energy called quanta or photons. Later, Albert Einstein applied this idea and suggested that light consists of quantized packets or photons. The document also discusses how Louis de Broglie applied wave-particle duality to electrons in 1924, showing that electrons can exhibit wave properties. This led to the development of quantum mechanics and the modern quantum mechanical model of the atom, which uses orbitals and quantum numbers to describe where electrons are likely to be found.Thermo#2



Thermo#2gbsliebs2002
Ã˝
This document defines different types of systems and their interactions with surroundings in thermodynamics. An open system can exchange both matter and energy with surroundings, a closed system can only exchange energy, and an isolated system cannot exchange either. Exothermic processes release heat to surroundings while endothermic processes absorb heat from surroundings. Specific examples of endothermic processes that require energy input include vaporization and melting, while freezing is exothermic and releases energy.Thermo#1



Thermo#1gbsliebs2002
Ã˝
Energy exists in two main forms - kinetic energy, which is energy due to motion, and potential energy, which is stored energy due to position. The total energy of a system is the sum of its kinetic and potential energies, and the total energy of the universe remains constant according to the law of conservation of energy. Heat is a transfer of energy between objects due to a temperature difference, while temperature measures the average kinetic energy of particles. The amount of heat required to change an object's temperature depends on its heat capacity, which relates the heat supplied to the temperature change.Gases Pt.1



Gases Pt.1gbsliebs2002
Ã˝
Pressure is force per unit area measured in pounds per square inch (psi) or pascals (Pa). Dalton's law of partial pressures states that the total pressure of a gas mixture equals the sum of the individual gas partial pressures. Boyle's law describes the inverse relationship between the pressure and volume of a gas at a constant temperature. Charles' law states that the volume of a gas increases or decreases proportionally with an increase or decrease in temperature when pressure remains constant.Stoichiometric Calculations



Stoichiometric Calculationsgbsliebs2002
Ã˝
The document discusses stoichiometric calculations and limiting reactants. It explains that stoichiometric calculations use the mole ratios from balanced chemical equations to determine the amount of reactants and products. The limiting reactant is the one that limits the amount of product that can be formed. The theoretical yield is the maximum amount of product possible based on the stoichiometry, while the actual yield is the amount experimentally obtained. Percent yield compares the actual and theoretical yields.Balancing Equations



Balancing Equationsgbsliebs2002
Ã˝
This document provides information on different types of chemical reactions:
1) Balancing equations must follow the law of conservation of mass and changing coefficients to balance atoms. Hydrogen and oxygen should be balanced last.
2) Synthesis reactions combine reactants to form a product. Decomposition reactions break compounds into simpler substances. Single replacement reactions involve one element replacing another in a compound.
3) Double replacement reactions involve the exchange of ions between reactants to form new ionic compounds as products. Products are determined by pairing ions that are "across" from each other.Moles



Molesgbsliebs2002
Ã˝
The document discusses moles, which are a unit used to measure very large quantities in chemistry. A mole is defined as 6.02 x 1023 particles, such as atoms, molecules, ions. This number is known as Avogadro's number. The document provides examples of how many everyday items would equal a mole to demonstrate just how large a mole is. It also discusses how moles can be used to convert between the number of particles, moles, and mass in grams.Periodic Table



Periodic Tablegbsliebs2002
Ã˝
Dmitri Mendeleev arranged the elements in the periodic table based on their chemical properties and left spaces for elements that were yet to be discovered. He was able to predict properties of these unknown elements accurately, including scandium, gallium, and germanium, which were later discovered with properties matching his predictions.Density
- 1. DensityMeasures how much matter (mass) is in a certain amount of space (volume)
- 2. Calculating DensityDensity equals mass divided by volumeD = m / vg/mL or g/cm3
- 3. Sample Problem #1What is the density of a chocolate bar that has a mass of 55.6 g and a volume of 30 cm3 ?
- 4. Sample #2What is the mass of a gold brick that has a density of 19.3 g/mL and a volume of 15 mL?
- 5. Sample #3What is the volume of silver that has a density of 10.5 g/cm3 and a mass of 25 g
- 6. Applying DensityWhy do some objects float in water while others sink?