Bacterial Endotoxin Testing_Basic_Presentation.ppt
- 1. Introduction to Bacterial Endotoxins Test Bio Solutions
- 2. Bacterial Endotoxins Test (BET) LAL ŌĆō Limulus Amebocyte Lysate (Limulus polyphemus) TAL ŌĆō Tachypleus Amebocyte Lysate (USP 29 only) (Tachypleus tridentatus, Tachypleus gigas) CAL ŌĆō Carcinoscropius Amebocyte Lysate (Carcinoscropius rotundicauda)
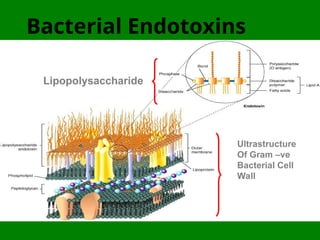
- 7. Bacterial Endotoxins Ultrastructure Of Gram ŌĆōve Bacterial Cell Wall Lipopolysaccharide
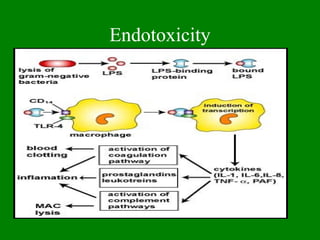
- 8. Endotoxicity
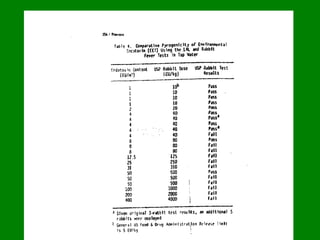
- 9. Rabbit Pyrogen test From 1940ŌĆÖs Rabbit Pyrogen test was method of choice for detection of pyrogen. This Rabbit pyrogen test was incorporated into USP XII in 1942.
- 10. Accidental Discovery Of LAL ŌĆó Dr. Frederick Bang ŌĆó Dr.Jack Levin
- 12. Reference Standard Endotoxin ŌĆó In 1974 first Reference Standard endotoxin was prepared Dr. J. A. Rudbach from Escherichia coli 0113:H110:K. ŌĆó In 1976 Lot EC ŌĆō 1 was prepared.
- 13. ŌĆó Currently EC - 6 (FDA RSE) or Lot G (USP RSE) ŌĆó Potency 10,000 EU/ vial
- 14. ŌĆó In 1980 USP XX included BET using LAL ŌĆó In 1987 the FDA published The Guideline on validation of the LAL test as an end-product endotoxin test for human and animal parenteral drugs, biological products, and medical devices.
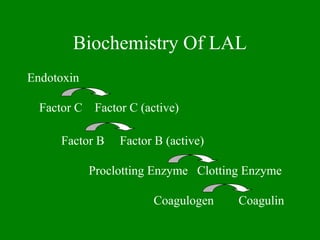
- 15. Biochemistry Of LAL Endotoxin Factor C Factor C (active) Factor B Factor B (active) Proclotting Enzyme Clotting Enzyme Coagulogen Coagulin
- 16. Procedure For LAL Test 100’üŁL of sample + 100’üŁL of LAL reagent 10X75 mm tubes 37’é▒ 1’é░ C 60 ’é▒2 min look for Gel formation after inverting tube by 180 ’é░
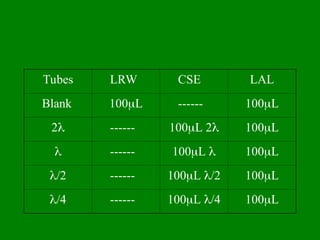
- 17. Reagents ŌĆó Lyophilized Wako LAL reagent, Sensitivity (’ü¼ EU/ml) ŌĆó Control Standard Endotoxin (CSE), for Control Curve and PPC* ŌĆó LAL Reagent Water (LRW), Diluent
- 18. Accessories ŌĆó Vortex Mixer ŌĆó Heating Block ŌĆó Depyrogenated Glass Test Tubes for Assay (10X75mm) ŌĆó Depyrogenated Glass Test Tubes for Dilutions ŌĆó Micro Pipette ŌĆó Sterile Micropipette Tips ŌĆó Timer
- 19. Initial Quality Control ŌĆó Analyst Qualification ŌĆó Verification of Testing accessories ŌĆó Verification of label claim sensitivity
- 20. As per USP : ŌĆó New Batch of LAL Reagent ŌĆó Any Change in Experimental Conditions ŌĆó Four Standards Concentrations in Quadruplicates with Negative Controls
- 21. ŌĆó Reconstitute Control Standard Endotoxin (CSE) referring to CoA. ŌĆó Dilute the CSE to 2’ü¼, ’ü¼, ’ü¼ /2 and ’ü¼ /4 EU/mL concentrations. Where ’ü¼ is sensitivity of LAL reagent
- 22. Tubes LRW CSE LAL Blank 100’üŁL ------ 100’üŁL 2’ü¼ ------ 100’üŁL 2’ü¼ 100’üŁL ’ü¼ ------ 100’üŁL ’ü¼ 100’üŁL ’ü¼/2 ------ 100’üŁL ’ü¼/2 100’üŁL ’ü¼/4 ------ 100’üŁL ’ü¼/4 100’üŁL
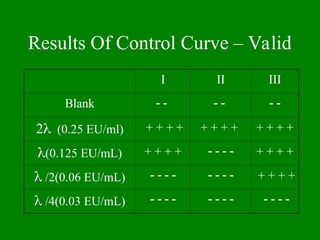
- 23. Results Of Control Curve ŌĆō Valid I II III Blank ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ 2’ü¼ (0.25 EU/ml) + + + + + + + + + + + + ’ü¼(0.125 EU/mL) + + + + ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ + + + + ’ü¼ /2(0.06 EU/mL) ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ + + + + ’ü¼ /4(0.03 EU/mL) ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ
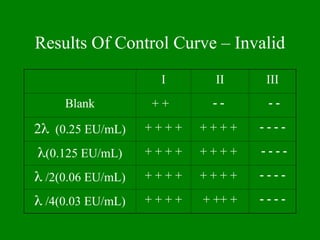
- 24. Results Of Control Curve ŌĆō Invalid I II III Blank + + ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ 2’ü¼ (0.25 EU/mL) + + + + + + + + ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ü¼(0.125 EU/mL) + + + + + + + + ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ü¼ /2(0.06 EU/mL) + + + + + + + + ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ü¼ /4(0.03 EU/mL) + + + + + ++ + ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ
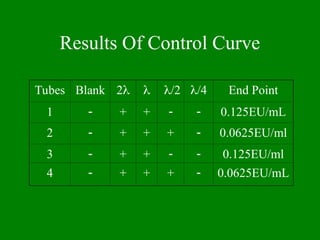
- 25. Results Of Control Curve Tubes Blank 2’ü¼ ’ü¼ ’ü¼/2 ’ü¼/4 End Point 1 ’ĆŁ + + ’ĆŁ ’ĆŁ 0.125EU/mL 2 ’ĆŁ + + + ’ĆŁ 0.0625EU/ml 3 ’ĆŁ + + ’ĆŁ ’ĆŁ 0.125EU/ml 4 ’ĆŁ + + + ’ĆŁ 0.0625EU/mL
- 26. Calculation Of Geometric Mean ŌĆó Formula : GM end point Concentration = antilog(’āźe/ŲÆ) Where, ’āźe = Sum of log of Endpoint Concentrations ŲÆ = Number of Replicates ŌĆó Calculation : GM = log (0.125) + log (0.125) + log (0.0625) + log (0.0625) 4 = Anti[(-0.9030) + (-0.9030) + (-1.2041)+ (-1.2041)] 4 = Antilog [(-1.0536)] = 0.0883 EU/mL ŌĆó Label Sensitivity is Confirmed if GM endpoint is between 2’ü¼ and ’ü¼/2
- 27. Endotoxin Limit ( EL) FDA established Endotoxin Limits based on Formula EL = K ( Tolerance limit ) M (maximum dose/ Kg/Hr) EL represents the maximum safe amount of endotoxin.
- 28. Endotoxin Limit ( EL) ŌĆó K = 5 EU/Kg Body weight for parenteral drugs except those administered intrathecally. 0.2 EU/kg for intrathecal drugs
- 29. Endotoxin Limit ( EL) M = Maximum dose administered to a patient per kg Body weight, per hour (no heroic dose).
- 30. Endotoxin Limit ( EL) ŌĆó The limit formula for radio pharmaceuticals is 175/V except for intrathecally administered products. 14/V for intrathecal drugs. V equals the maximum recommended dose, in mL, at the expiration date or time. ŌĆó For drugs administered on a per Square Meter of Body Surface: 2.5 EU/ [ ( dose * 1.8 sq.. m.)/ 70 Kg]
- 31. Product Testing Negative Product Control (NPC) - Sample + LAL Positive Product Control (PPC) - Sample + CSE (2’ü¼) + LAL Negative Water Control (NWC) - LRW + LAL Positive Water Control (PWC) - LRW + CSE (2’ü¼) + LAL
- 32. Interference Significant difference between the end points of positive water control and positive product control using standard endotoxin.
- 33. Interference ŌĆó Sub optimal pH concentration ŌĆó Endotoxin modification ŌĆó Container effects ŌĆó Unbalanced cation levels ŌĆó Protein or Enzyme modification
- 34. Maximum Valid Dilution (MVD) (Potency of the product) x (Endotoxin Limit) MVD = __________________________________ ’ü¼ Potency of product = Concentration of the product in units/mL Endotoxin Limit = K/M lambda (’ü¼ ) = Lysate label claim sensitivity
- 35. Example Drug : Cefotaxime Sodium Sterile 500mg/2mL Endotoxin Limit : NMT 0.2 EU/mg Lysate sensitivity ( ’ü¼ ) : = 0.125 EU/mL MVD = Conc. of Drug (potency) x E.L ’ü¼ = 250mg/ mL X 0.2 EU/mg 0.125EU/mL MVD = 400
- 36. Minimum Valid Concentration (MVC) ŌĆó MVC = ’ü¼ / E.L.
- 37. Example Drug : Cefotaxime Sodium Sterile Endotoxin Limit : NMT 0.2 EU/mg Lysate sensitivity : 0.125 EU/mL MVC = ’ü¼ / E.L. = 0.125 EU/mL 0.2EU/mg = 0.625mg/ mL
- 38. Product Validation ŌĆó The validation for LAL compatibility of a drug product is a test condition where an endotoxin standard is detected with the same efficiency in a test sample as it is in LRW.
- 39. Product Validation Phase I: Preliminary Screening Determines the Non Interfering Dilution / Concentration of the product which is used for actual validation. Phase II: Product Validation
- 40. Phase I: Preliminary Screening ŌĆó Prepare dilutions of product up to MVD such as, MVD / 16, MVD / 8, MVD / 4, MVD / 2, MVD ŌĆó Run NPC AND PPC ŌĆó Least DILUTION / highest CONC. of product for which PPC is Positive and NPC is Negative is the Non Interfering Dilution (NID)/ Non Interfering Concentration (NIC).
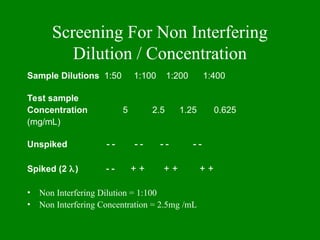
- 41. Screening For Non Interfering Dilution / Concentration Sample Dilutions 1:50 1:100 1:200 1:400 Test sample Concentration 5 2.5 1.25 0.625 (mg/mL) Unspiked ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ Spiked (2 ’ü¼) ’ĆŁ ’ĆŁ + + + + + + ŌĆó Non Interfering Dilution = 1:100 ŌĆó Non Interfering Concentration = 2.5mg /mL
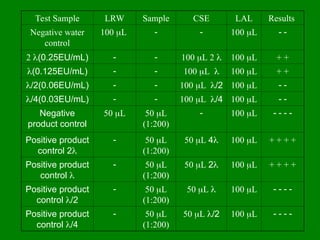
- 42. Test Sample LRW Sample CSE LAL Results Negative water control 100 ’üŁL ’ĆŁ ’ĆŁ 100 ’üŁL ’ĆŁ ’ĆŁ 2 ’ü¼(0.25EU/mL) ’ĆŁ ’ĆŁ 100 ’üŁL 2 ’ü¼ 100 ’üŁL + + ’ü¼(0.125EU/mL) ’ĆŁ ’ĆŁ 100 ’üŁL ’ü¼ 100 ’üŁL + + ’ü¼/2(0.06EU/mL) ’ĆŁ ’ĆŁ 100 ’üŁL ’ü¼/2 100 ’üŁL ’ĆŁ ’ĆŁ ’ü¼/4(0.03EU/mL) ’ĆŁ ’ĆŁ 100 ’üŁL ’ü¼/4 100 ’üŁL ’ĆŁ ’ĆŁ Negative product control 50 ’üŁL 50 ’üŁL (1:200) ’ĆŁ 100 ’üŁL ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ Positive product control 2’ü¼ ’ĆŁ 50 ’üŁL (1:200) 50 ’üŁL 4’ü¼ 100 ’üŁL + + + + Positive product control ’ü¼ ’ĆŁ 50 ’üŁL (1:200) 50 ’üŁL 2’ü¼ 100 ’üŁL + + + + Positive product control ’ü¼/2 ’ĆŁ 50 ’üŁL (1:200) 50 ’üŁL ’ü¼ 100 ’üŁL ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ Positive product control ’ü¼/4 ’ĆŁ 50 ’üŁL (1:200) 50 ’üŁL ’ü¼/2 100 ’üŁL ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ
- 43. Geometric Mean Calculation GM end point Concentration = antilog(’āźe/ŲÆ) GM in Water = log (0.125) + log (0.125) + log (0.125) + log( 0.125) 4 = Anti[(-0.9030) + (-0.9030) +(-0.9030) + (-0.9030)] 4 = Antilog [(-0.9030)] = 0.125 EU/mL GM in Product = log (0.125) + log (0.125) + log (0.125) + log( 0.125) 4 = Anti[(-0.9030) + (-0.9030) +(-0.9030) + (-0.9030)] 4 = Antilog [(-0.9030)] = 0.125 EU/mL
- 44. If endpoint of CSE in product is within ’é▒ one two fold of endpoint in water ŌĆō Product is Validated
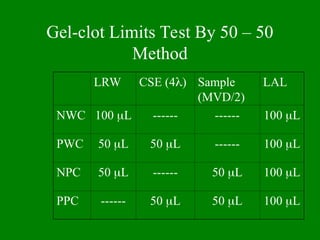
- 45. Gel-clot Limits Test By 50 ŌĆō 50 Method LRW CSE (4’ü¼) Sample (MVD/2) LAL NWC 100 ’üŁL ------ ------ 100 ’üŁL PWC 50 ’üŁL 50 ’üŁL ------ 100 ’üŁL NPC 50 ’üŁL ------ 50 ’üŁL 100 ’üŁL PPC ------ 50 ’üŁL 50 ’üŁL 100 ’üŁL
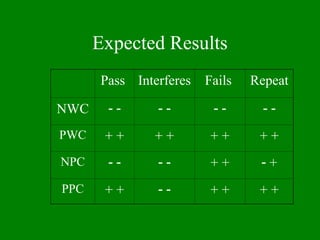
- 46. Expected Results Pass Interferes Fails Repeat NWC ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ PWC + + + + + + + + NPC ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ + + ’ĆŁ + PPC + + ’ĆŁ ’ĆŁ + + + +
- 47. Applications ŌĆó Large Volume Parenterals (LVPs) ŌĆó Multiple - ingredient drugs ŌĆó Small Volume Parenterals (SVPs) ŌĆó Radiopharmaceuticals ŌĆó Biologicals ŌĆó Water system validation ŌĆó Validation of Dry heat Sterilizer ŌĆó Medical devices

















![Calculation Of Geometric Mean
ŌĆó Formula :
GM end point Concentration = antilog(’āźe/ŲÆ)
Where,
’āźe = Sum of log of Endpoint Concentrations
ŲÆ = Number of Replicates
ŌĆó Calculation :
GM = log (0.125) + log (0.125) + log (0.0625) + log (0.0625)
4
= Anti[(-0.9030) + (-0.9030) + (-1.2041)+ (-1.2041)]
4
= Antilog [(-1.0536)]
= 0.0883 EU/mL
ŌĆó Label Sensitivity is Confirmed if GM endpoint is between 2’ü¼ and ’ü¼/2](https://image.slidesharecdn.com/betbasicpresentation-250330084023-5155ef48/85/Bacterial-Endotoxin-Testing_Basic_Presentation-ppt-26-320.jpg)



![Endotoxin Limit ( EL)
ŌĆó The limit formula for radio pharmaceuticals is
175/V except for intrathecally administered
products. 14/V for intrathecal drugs.
V equals the maximum recommended dose, in mL,
at the expiration date or time.
ŌĆó For drugs administered on a per Square Meter of
Body Surface:
2.5 EU/ [ ( dose * 1.8 sq.. m.)/ 70 Kg]](https://image.slidesharecdn.com/betbasicpresentation-250330084023-5155ef48/85/Bacterial-Endotoxin-Testing_Basic_Presentation-ppt-30-320.jpg)










![Geometric Mean Calculation
GM end point Concentration = antilog(’āźe/ŲÆ)
GM in Water = log (0.125) + log (0.125) + log (0.125) + log( 0.125)
4
= Anti[(-0.9030) + (-0.9030) +(-0.9030) + (-0.9030)]
4
= Antilog [(-0.9030)]
= 0.125 EU/mL
GM in Product = log (0.125) + log (0.125) + log (0.125) + log( 0.125)
4
= Anti[(-0.9030) + (-0.9030) +(-0.9030) + (-0.9030)]
4
= Antilog [(-0.9030)]
= 0.125 EU/mL](https://image.slidesharecdn.com/betbasicpresentation-250330084023-5155ef48/85/Bacterial-Endotoxin-Testing_Basic_Presentation-ppt-43-320.jpg)