C3
- 2. ปฏิกิริยาการตรึงคาร์บอนไดออกไซด์ (CO2 fixation หรือ Dark reaction หรือ Calvin cycle) การทดลองของเมลวิน คัลวิน (Melvin Calvin) และแอนดรู เอ เบนสัน คลอเรลลา เติม CO2 ในรูปของ H14CO3- ลิ้นควบคุมการปิดเปิดแสง เทอร์โมมิเตอร์ ภาชนะเก็บคลอเรลลาเป็นระยะ ภายในบรรจุเมทานอลที่ร้อน เพื่อนาคลอเรลลาทันที สารละลาย NaHCO3
- 3. เมื่อเวลาผ่านไป - 1 นาที ตรวจสารประกอบแล้วพบ 14C ใน สารประกอบหลาย ชนิดรวมทั้งน้าตาลกลูโคส (สาร C 3,C5,C6) เช่น (สาร C3 = PGA ,C5 = RuBP,C6 = กลูโคส) - แต่เมื่อให้การสังเคราะห์แสงในระยะเวลาสั้นลง ประมาณ 2 วินาที ตรวจพบ 14C อยู่ในสสาร ประกอบที่มีคาร์บอน 3 อะตอม (PGA)
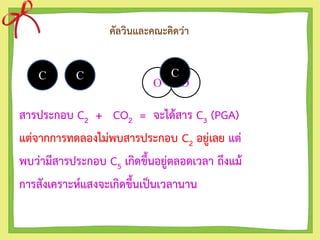
- 4. คัลวินและคณะคิดว่า สารประกอบ C2 + CO2 = จะได้สาร C3 (PGA) แต่จากการทดลองไม่พบสารประกอบ C2 อยู่เลย แต่ พบว่ามีสารประกอบ C5 เกิดขึ้นอยู่ตลอดเวลา ถึงแม้ การสังเคราะห์แสงจะเกิดขึ้นเป็นเวลานาน C C O O C
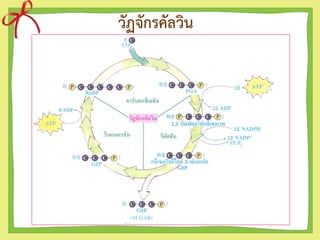
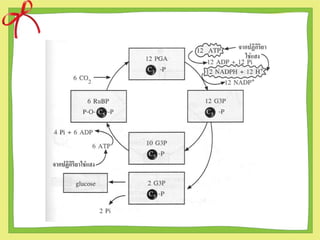
- 5. ดังนั้นนักวิทยาศาสตร์จึงได้ตั้งสมมติฐานขึ้นว่า สาร C5 คงจะรวมกับ CO2 ได้สารประกอบชนิด ใหม่ เป็นสาร C6 เพราะ RuBP(สาร C5 รวมกับ สาร C1 (CO2 ) แต่สารนี้ไม่อยู่ตัวจะสลายต่อให้ สารประกอบ C3 คือ PGA 2 โมเลกุล ดังที่จะได้ศึกษาวัฏจักรของคัลวิน
- 6. The step of Calvin cycle 1. Carbon fixation 2. Reduction 3. Regeneration
- 11. ข้อสังเกตของวัฏจักรคัลวิน 1. โดยปกติวัฏจักรคัลวินจะต้องเกิด 2 รอบจึงจะได้ PGAL 2 โมเลกุล ซึ่งเพียงพอต่อการสร้างกลูโคส (6C)1 โมเลกุล 2. ในพืชทั่วๆไป สารชนิดแรกที่คงตัวที่ได้จากการตรึง CO2 คือ PGA ซึ่งมี 3C จึงเรียกการตรึง CO2 แบบนี้ว่า C3-pathway และเรียกว่าพืช C-3 ซึ่งก็คือพืชใบเลี้ยงคู่และใบเลี้ยงเดี่ยวทั่วๆ ไป (ข้าวสาลี,ถั่วเหลือง)
- 15. ‡∏Ň∏£‡∏∞‡∏ö‡∏߇∏ô‡∏Ň∏≤‡∏£‡∏™‡∏±‡∏á‡πć∏ч∏£‡∏≤‡∏∞‡∏´‡πå‡∏î‡πâ‡∏߇∏¢‡πŇ∏™‡∏á‡∏LJ∏≠‡∏á‡∏û‡∏∑‡∏ä ‡∏Ň∏£‡∏∞‡∏ö‡∏߇∏ô‡∏Ň∏≤‡∏£‡∏™‡∏±‡∏á‡πć∏ч∏£‡∏≤‡∏∞‡∏´‡πå‡∏î‡πâ‡∏߇∏¢‡πŇ∏™‡∏á‡∏LJ∏≠‡∏á‡∏û‡∏∑‡∏ä ‡∏õ‡∏£‡∏∞‡∏Ň∏≠‡∏ö‡∏î‡πâ‡∏߇∏¢ 2 ‡∏™‡πà‡∏߇∏ô‡πɇ∏´‡∏ç‡πà ‡∏ч∏∑‡∏≠ 1. ‡∏Ň∏£‡∏∞‡∏ö‡∏߇∏ô‡∏Ň∏≤‡∏£‡πć∏õ‡∏•‡∏µ‡πà‡∏¢‡∏ô‡∏û‡∏•‡∏±‡∏á‡∏á‡∏≤‡∏ô‡πŇ∏™‡∏á‡πɇ∏´‡πâ‡πć∏õ‡πá‡∏ô‡∏û‡∏•‡∏±‡∏á‡∏á‡∏≤‡∏ô‡π∂ƒ‡∏ч∏°‡∏µ ‡πLJ∏î‡∏¢‡∏Ň∏≤‡∏£‡∏™‡∏£‡πâ‡∏≤‡∏á ATP ‡πŇ∏•‡∏∞ NADPH ‡∏î‡πâ‡∏߇∏¢‡∏õ‡∏è‡∏¥‡∏Ň∏¥‡∏£‡∏¥‡∏¢‡∏≤‡πŇ∏™‡∏á 2. ‡∏Ň∏≤‡∏£‡∏ô‡∏≤ ATP ‡πŇ∏•‡∏∞ NADPH ‡πч∏õ‡πɇ∏ä‡πâ‡πɇ∏ô‡∏õ‡∏è‡∏¥‡∏Ň∏¥‡∏£‡∏¥‡∏¢‡∏≤‡∏Ň∏≤‡∏£‡∏ï‡∏£‡∏∂‡∏á CO2 ‡πć∏û‡∏∑‡πà‡∏≠‡∏™‡∏£‡πâ‡∏≤‡∏á‡∏™‡∏≤‡∏£‡∏õ‡∏£‡∏∞‡∏Ň∏≠‡∏ö‡∏ч∏≤‡∏£‡πå‡πLJ∏ö‡πч∏Ƈπć∏î‡∏£‡∏ï
- 16. • คือ กระบวนการที่พืชมีการตรึงออกซิเจนและคายคาร์บอนไดออกไซด์ ในขณะที่พืชได้รับแสง • กระบวนการโฟโตเรสไพเรชัน ต่างจากการหายใจหรือการสลายสารอาหาร เพราะโฟโตเรสไพเรชันเกิดขึ้นเฉพาะเซลล์ที่มีคลอโรพลาสต์เท่านั้น • การตรึง CO2 และการตรึง O2 ดาเนินไปพร้อมๆ กันในอัตราส่วน 3 ต่อ 1 • โฟโตเรสไพเรชันช่วยป้องกันความเสียหายแก่ระบบการสังเคราะห์ด้วยแสง เช่น กรณีที่พืชได้รับแสงมากแต่มีปริมาณ CO2 น้อย Photorespiration
- 17. Photorespiration การตรึง CO2 ของ RuBP ต้องใช้เอนไซม์ Rubisco ซึ่งอยู่ใน สโตรมาของคลอโรพลาสต์ เอนไซม์นี้นอกจากกระตุ้นให้ RuBP ตรึง CO2 แล้วยังตรึง O2 ได้ด้วย เมื่อพืช ตรึง O2 ด้วย RuBP ซึ่ง RuBP จะถูกสลายเป็นสารประกอบ คาร์บอน 2 อะตอม เพราะฉะนั้นการจะสร้าง RuBP จึงเสีย CO2 ไปบางส่วน การตรึง O2 และคาย CO2 ในเวลาที่พืชได้รับแสง จึงเรียกว่า โพโตเรสไพเรชัน (Photorespiration)