Ch8 study of compounds hydrogen chloride
Download as pptx, pdf1 like1,843 views
1. The document discusses the reactions of dilute and concentrated hydrochloric acid with various substances like magnesium, zinc oxide, sodium hydrogen carbonate, potassium permanganate, and manganese dioxide. 2. It also describes tests for identifying hydrochloric acid like its reactions with litmus, silver nitrate, and ammonia to produce white fumes. Safety precautions for preparing HCl in the laboratory are highlighted. 3. A series of questions related to the reactions and preparation of hydrochloric acid are presented along with their answers concerning the products formed and equations involved. Diagrams of apparatus used in experiments like the fountain experiment are also included.
1 of 39
Downloaded 23 times




















![Q10. The diagram shows a simple arrangement
of the fountain experiment: [3]
(i) Name the two gases you have studied which
can be used in this experiment.
Ans, Ammonia, hydrogen chloride
(i) What is the common property demonstrated
by this experiment?
High solubility of
ammonia or
hydrogen chloride
in water.](https://image.slidesharecdn.com/ch8studyofcompoundshydrogenchloride-200501121202/85/Ch8-study-of-compounds-hydrogen-chloride-28-320.jpg)











Ad
Recommended
Electrolysis
ElectrolysisKim B
Ėý
This document discusses electrolysis, which is the process of using direct current to cause non-spontaneous chemical reactions. Electrolysis requires an electrolyte containing free ions, a direct current power supply, and two electrodes. During electrolysis, ions are oxidized or reduced at the electrodes through electron transfer. As an example, electrolysis can be used to purify copper by dissolving impure copper and conducting electrolysis, depositing pure copper at the cathode. Electrolysis equations describe the half-reactions that occur at each electrode. The document also provides an example of electrolyzing brine to produce chlorine gas, hydrogen gas, and sodium hydroxide._A_ Hydrogen Chloride.pptx
_A_ Hydrogen Chloride.pptxPratikVirSingh1
Ėý
This document provides information about the study of the compound hydrogen chloride. It discusses the preparation of hydrogen chloride gas from sodium chloride, including the laboratory method and reaction. It also describes experiments to show the density and solubility of hydrogen chloride gas. The preparation of hydrochloric acid by dissolving hydrogen chloride gas in water is explained. The physical and chemical properties of both hydrogen chloride gas and hydrochloric acid are outlined. Uses of hydrochloric acid include industrial applications and as a medicine. Tests for identifying hydrogen chloride gas and hydrochloric acid involve reactions with ammonia and silver nitrate that produce precipitates.Ch ammonia
Ch ammoniaRajiv Jain
Ėý
This document discusses ammonia, its properties, production, and reactions. It provides information on collecting ammonia through downward displacement of air and drying it with calcium oxide. Key reactions discussed include producing ammonia from ammonium chloride and calcium hydroxide, and the reducing nature of ammonia shown through its reaction with copper oxide. The Haber and Ostwald processes for producing ammonia and nitric acid respectively are summarized.GAY-LUSSACS-PPT.ppt
GAY-LUSSACS-PPT.pptFrancis de Castro
Ėý
This document discusses Gay-Lussac's law, which states that for a fixed amount of gas kept at constant volume, the pressure and temperature are directly proportional. An example problem demonstrates how to use the law to calculate the pressure of a gas in an aerosol can if the temperature increased dramatically from being thrown on a fire. The document also provides an example of how Gay-Lussac's law allows pressure cookers to cook food faster by trapping steam at higher pressures and temperatures than normal cooking.Ventilation and Perfusion in different zones of lungs.
Ventilation and Perfusion in different zones of lungs.Gyaltsen Gurung
Ėý
The document discusses the definitions and classifications of ventilation and perfusion in the lungs, including the ventilation/perfusion ratio and its correlation with pulmonary tuberculosis. It outlines different zones of lung perfusion and ventilation, providing mathematical calculations for each zone. The findings indicate that the apical zone is more prone to pulmonary tuberculosis due to lower perfusion and higher ventilation/perfusion ratios.Chemical kinetics- Physical Chemistry
Chemical kinetics- Physical ChemistrySanchit Dhankhar
Ėý
The document discusses chemical kinetics, focusing on reaction rates, factors affecting them, and rate laws. It explains how reaction rates are determined, including average and instantaneous rates, as well as the concepts of molecularity and order of reactions. Additionally, it covers integrated rate equations and methods for determining the order of a reaction through various techniques.Refraction of light in lenses
Refraction of light in lensesMartinGeraldine
Ėý
A lens is a transparent piece of glass with curved refracting surfaces that can be either convex or concave. Convex lenses are thicker in the middle and focus parallel rays of light to a focal point by converging the rays. Concave lenses are thinner in the middle and cause parallel rays of light to diverge as if originating from a focal point behind the lens. Lenses are used to either focus or diverge light depending on whether they are convex or concave.ICSE English Language Class X Handwritten Notes
ICSE English Language Class X Handwritten NotesGauri S
Ėý
This document contains handwritten notes for the ICSE Class X English Language course. The notes cover various topics related to the English language such as grammar, composition, comprehension and literature. The handwritten nature of the notes suggests they were taken by a student in class to aid in learning the material.Air and water
Air and waterCarole Paquette
Ėý
The document provides information about various chemistry concepts related to air and water:
- It describes chemical tests to identify water and the purification of water supplies through filtration and chlorination.
- The composition of clean air is described as 78% nitrogen, 21% oxygen and small quantities of other gases. Common air pollutants like carbon monoxide and their sources are stated.
- Fractional distillation is outlined as the process used to separate oxygen and nitrogen from liquid air based on their different boiling points.
- Rusting is described as a reaction between iron, air and water that can be prevented by methods like painting and galvanizing to exclude oxygen.Salts and their preparation power point
Salts and their preparation power pointTimothy Kwando
Ėý
Salts are formed when hydrogen ions in acids are replaced by metal ions or ammonium ions. There are two main types of salts - normal salts that do not contain replaceable hydrogen and acidic salts that contain replaceable hydrogen. Insoluble salts are prepared through precipitation reactions by mixing solutions of reactants containing the ions of the insoluble salt. Soluble salts can be prepared through filtration and crystallization using excess insoluble reactants or through titration using exact quantities of reactants.Acids, Bases and Salts (Chemistry 'O' level)
Acids, Bases and Salts (Chemistry 'O' level)Faiz Abdullah
Ėý
The document provides a comprehensive overview of acids, bases, and salts, including definitions, properties, and reactions. It distinguishes between strong and weak acids and bases, describes various chemical reactions involving these compounds, and covers the concept of neutralization. Additionally, it discusses the solubility of salts and different methods for preparing them.Grade 10 Organic Chemistry
Grade 10 Organic ChemistryAlice Palmer
Ėý
1) Crude oil is a mixture of hydrocarbons formed from the remains of ancient plants and animals over millions of years under high pressure and temperature conditions underground.
2) Fractional distillation is used to separate crude oil into different hydrocarbon fractions like gasoline, kerosene and diesel in a fractionating column based on their boiling points.
3) Short hydrocarbon fractions are collected at the top of the fractionating column while longer fractions are collected at the bottom during the fractional distillation process.Chapter acids, bases and salts(class 10)
Chapter acids, bases and salts(class 10)PriyankaSoni127
Ėý
This document discusses acids, bases, and salts. It defines acids as substances that produce hydrogen ions (H+) in aqueous solution, making them sour and able to turn litmus red. Bases are defined as substances that produce hydroxide ions (OH-) in solution, making them soapy and able to turn litmus blue. Salts are formed by the reaction of acids and bases and can be acidic, basic, or neutral depending on the reactants. Common natural and synthetic acid-base indicators are also described. The document then discusses the properties and reactions of acids, bases, and salts and how pH is used to measure acidity. Finally, several industrial chemicals derived from sodium chloride (common salt) are summarized, includingGroup VII elements - Halogens
Group VII elements - HalogensGiiang
Ėý
Group VII elements are called halogens. They exist as diatomic molecules (F2, Cl2, Br2, I2) and have seven electrons in their outer shell. Fluorine has the smallest atomic radius while iodine has the largest due to more electron shells. Melting and boiling points decrease from fluorine to iodine due to weaker van der Waals forces between larger molecules. Electronegativity decreases from fluorine to iodine as the nucleus attracts electrons less. Halogens can gain electrons to form ions or share electrons to form covalent bonds. More reactive halogens can displace less reactive ones from solutions.Metal extraction slides
Metal extraction slidesfatimahbegum
Ėý
- Iron is extracted from its ore, haematite, in a blast furnace using coke, limestone, and hot air. The limestone removes impurities and produces slag.
- Iron rusts in the presence of oxygen and water to form iron(III) oxide. Rusting can be prevented by barriers like paint, grease, or sacrificial protection using more reactive metals like zinc.
- Recycling metals saves limited metal resources and reduces costs and pollution compared to extracting virgin metals. However, recycling has costs and challenges like obtaining scrap metals.Ch10 study of compounds nitric acid
Ch10 study of compounds nitric acidRajiv Jain
Ėý
This document summarizes key questions and answers from a YouTube chemistry lesson on nitric acid and its reactions with metals and other compounds.
The lesson covers the preparation of nitric acid, its reactions with copper and sulfur, and the identification and equations for reactions of nitric acid with compounds like copper carbonate, lead nitrate, potassium nitrate, and ammonium nitrate. Key reactions include the decomposition of nitric acid and metal nitrates like copper nitrate and lead nitrate when heated. Equations are provided for the oxidation of sulfur and copper by concentrated nitric acid.Oxides
OxidessYhira
Ėý
This document discusses different types of oxides based on their reactions with water and acids or bases. It defines acidic, basic, amphoteric, and neutral oxides. Acidic oxides react with water to form acids, while basic oxides react with acids to form salts and water. Amphoteric oxides can behave as either acidic or basic oxides depending on the reaction. Neutral oxides show no acidic or basic properties and are insoluble in water. The document provides examples of common oxides that fall into each category and an decision tree for classifying an unknown oxide based on its solubility properties.igcse ORGANIC CHEMISTRY PRESENTATION.pptx
igcse ORGANIC CHEMISTRY PRESENTATION.pptxdongleiwhu
Ėý
This document provides an overview of organic chemistry, including:
- Organic chemistry is the study of carbon-containing compounds and their properties/reactions.
- Organic compounds contain carbon and can be classified as hydrocarbons, containing only carbon and hydrogen, or other organic functional groups.
- Key concepts include functional groups, homologous series, structural formulas and structural isomers. Reaction types like addition, substitution and elimination are also covered.Reaction of metals with water
Reaction of metals with water AakankshaYaduvanshi
Ėý
This document discusses the reactions of various metals with water, steam, and heat. Some reactive metals like sodium and potassium react vigorously with cold water, producing metal hydroxides and hydrogen gas. The reaction of potassium with cold water produces a colorless solution and burns with a lilac color. Calcium also reacts with cold water but does not ignite the hydrogen produced. Magnesium reacts with hot water but not cold water. Less reactive metals like aluminum, iron and zinc only react with steam, producing metal oxides and hydrogen gas. Some metals like lead, copper, silver and gold do not react at all with water, steam or heat.Acid Bases and Salts and Chemical Equations
Acid Bases and Salts and Chemical EquationsSanchit Duseja
Ėý
1) The document discusses chemical reactions and equations, focusing on chemical changes, types of chemical equations, and balanced chemical equations.
2) It then covers the five main types of chemical reactions - combination, decomposition, displacement, double displacement, and oxidation-reduction reactions.
3) Finally, it discusses acids, bases, and salts. It defines acids and bases, lists their key properties, methods of classification, common examples, and uses.Salt preparation
Salt preparation Zainab Yahya
Ėý
The document discusses four methods for preparing salts: 1) Reacting a metal with an acid, 2) Reacting an insoluble base with an acid, 3) Neutralizing an alkali with an acid through titration, and 4) Precipitation. It then provides examples of soluble and insoluble compounds, and explains how to specifically prepare zinc sulfate by reacting zinc powder with sulfuric acid. The document asks to describe how to prepare several example salts using these methods.6.3 (a) electrolysis of an aqueous solution
6.3 (a) electrolysis of an aqueous solutionAzieda Dot
Ėý
The document discusses the electrolysis of aqueous solutions. It explains that during electrolysis, only one ion is selectively discharged at each electrode based on its position in the electrochemical series, the nature of the electrode, and the concentration of ions. The ion discharged at the anode depends on which is easier to oxidize, while the ion discharged at the cathode depends on which is easier to reduce. Different products are formed depending on these factors and the specific electrolyte used.Y11 Chem - 2. Reactivity Series.pptx
Y11 Chem - 2. Reactivity Series.pptxKristieCorpus
Ėý
The document discusses the reactivity series of metals and how it is determined. The reactivity of a metal is based on its tendency to lose electrons and form positive ions. Experiments are conducted to see which metals react with water, acids, carbon, other metals, and metal ion solutions. These experiments allow metals to be ranked in a reactivity series from most reactive to least reactive. The reactivity series can then be used to predict and understand redox reactions between metals.Unit 6 Organic Chemistry
Unit 6 Organic ChemistryHaihao Liu
Ėý
Organic chemistry revision notes cover the formation of fossil fuels like oil from dead marine organisms under heat and pressure. Crude oil is separated into fractions like gasoline and kerosene through fractional distillation, and combustion produces pollution like carbon monoxide and nitrogen oxides. The energy released during combustion can be measured using a calorimeter. Homologous series are families of compounds with the same functional group and general formula that differ by CH2. Main series include alkanes, alkenes, and alcohols, which are named based on their carbon chain and functional group.Unit 2, Lesson 2.8 - Acids, Bases, and Salts
Unit 2, Lesson 2.8 - Acids, Bases, and Saltsjudan1970
Ėý
The document discusses the properties and differences between acids, bases, and salts, including their behavior in water, chemical formulas, and applications in the human body and industry. It also covers concepts such as the pH scale, neutralization reactions, and classifications of acids and bases. Additional educational activities and a quiz preparation outline are included at the end.Chemical reactions of acids and bases
Chemical reactions of acids and basesVeenuGupta8
Ėý
The document explains the properties and behaviors of acids and bases. Acids yield hydrogen ions (H+) in water and dissociate to form hydronium ions, whereas bases provide hydroxide ions (OHâ). It also discusses neutralization reactions, the heat released during the dissolution of acids and bases in water, and the reactions of acids with metals and carbonates.Acids, bases and salts IGCSE Chemistry
Acids, bases and salts IGCSE Chemistry Maitreyee Joshi
Ėý
This document provides an introduction to acids, bases, and salts for GCSE chemistry students. It discusses key topics like acidity and alkalinity, indicators, the pH scale, types of acids including strong and weak acids, and various methods for making salts through reactions between acids and metals, metal oxides, metal carbonates, metal hydroxides, and ammonia. The document is intended to help students understand and revise these core chemistry concepts in preparation for their exams.How to write a chemical formula
How to write a chemical formulaZahraFazal6
Ėý
This document discusses how to write chemical formulas. It explains that a chemical formula tells us the types and numbers of atoms in a compound. It then outlines five steps for writing chemical formulas: 1) Write the symbols of the ions side by side in order of positive then negative, 2) Write the valency of each ion, 3) Transfer the valencies to offset them, 4) Omit valencies that are the same, and include different valencies in the formula, 5) For radicals like sulfate and phosphate, the net charge indicates the radical's valency and it is written in parentheses.chlorine.pdf
chlorine.pdfBagalanaSteven
Ėý
Chlorine and its compounds are discussed. Chlorine can be prepared in the laboratory by reacting concentrated hydrochloric acid with manganese(IV) oxide or potassium manganate(VII). Chlorine is a greenish-yellow gas that is denser than air and acts as a strong oxidizing agent and bleach. It reacts with many metals and non-metals to form chlorides. Common uses of chlorine include water disinfection and manufacturing of bleaches, plastics and pesticides.Ch3 acid bases and salts
Ch3 acid bases and saltsRajiv Jain
Ėý
This document summarizes a YouTube chemistry video about acids, bases, and salts. It discusses the pH of salt solutions, methods of preparing salts, and properties of different salts like solubility and water of crystallization. It also contains sample chemistry questions and their answers about identifying oxides, salts, and writing chemical equations. Key topics covered include acid-base reactions, salt preparations, and properties of common salts and oxides.More Related Content
What's hot (20)
Air and water
Air and waterCarole Paquette
Ėý
The document provides information about various chemistry concepts related to air and water:
- It describes chemical tests to identify water and the purification of water supplies through filtration and chlorination.
- The composition of clean air is described as 78% nitrogen, 21% oxygen and small quantities of other gases. Common air pollutants like carbon monoxide and their sources are stated.
- Fractional distillation is outlined as the process used to separate oxygen and nitrogen from liquid air based on their different boiling points.
- Rusting is described as a reaction between iron, air and water that can be prevented by methods like painting and galvanizing to exclude oxygen.Salts and their preparation power point
Salts and their preparation power pointTimothy Kwando
Ėý
Salts are formed when hydrogen ions in acids are replaced by metal ions or ammonium ions. There are two main types of salts - normal salts that do not contain replaceable hydrogen and acidic salts that contain replaceable hydrogen. Insoluble salts are prepared through precipitation reactions by mixing solutions of reactants containing the ions of the insoluble salt. Soluble salts can be prepared through filtration and crystallization using excess insoluble reactants or through titration using exact quantities of reactants.Acids, Bases and Salts (Chemistry 'O' level)
Acids, Bases and Salts (Chemistry 'O' level)Faiz Abdullah
Ėý
The document provides a comprehensive overview of acids, bases, and salts, including definitions, properties, and reactions. It distinguishes between strong and weak acids and bases, describes various chemical reactions involving these compounds, and covers the concept of neutralization. Additionally, it discusses the solubility of salts and different methods for preparing them.Grade 10 Organic Chemistry
Grade 10 Organic ChemistryAlice Palmer
Ėý
1) Crude oil is a mixture of hydrocarbons formed from the remains of ancient plants and animals over millions of years under high pressure and temperature conditions underground.
2) Fractional distillation is used to separate crude oil into different hydrocarbon fractions like gasoline, kerosene and diesel in a fractionating column based on their boiling points.
3) Short hydrocarbon fractions are collected at the top of the fractionating column while longer fractions are collected at the bottom during the fractional distillation process.Chapter acids, bases and salts(class 10)
Chapter acids, bases and salts(class 10)PriyankaSoni127
Ėý
This document discusses acids, bases, and salts. It defines acids as substances that produce hydrogen ions (H+) in aqueous solution, making them sour and able to turn litmus red. Bases are defined as substances that produce hydroxide ions (OH-) in solution, making them soapy and able to turn litmus blue. Salts are formed by the reaction of acids and bases and can be acidic, basic, or neutral depending on the reactants. Common natural and synthetic acid-base indicators are also described. The document then discusses the properties and reactions of acids, bases, and salts and how pH is used to measure acidity. Finally, several industrial chemicals derived from sodium chloride (common salt) are summarized, includingGroup VII elements - Halogens
Group VII elements - HalogensGiiang
Ėý
Group VII elements are called halogens. They exist as diatomic molecules (F2, Cl2, Br2, I2) and have seven electrons in their outer shell. Fluorine has the smallest atomic radius while iodine has the largest due to more electron shells. Melting and boiling points decrease from fluorine to iodine due to weaker van der Waals forces between larger molecules. Electronegativity decreases from fluorine to iodine as the nucleus attracts electrons less. Halogens can gain electrons to form ions or share electrons to form covalent bonds. More reactive halogens can displace less reactive ones from solutions.Metal extraction slides
Metal extraction slidesfatimahbegum
Ėý
- Iron is extracted from its ore, haematite, in a blast furnace using coke, limestone, and hot air. The limestone removes impurities and produces slag.
- Iron rusts in the presence of oxygen and water to form iron(III) oxide. Rusting can be prevented by barriers like paint, grease, or sacrificial protection using more reactive metals like zinc.
- Recycling metals saves limited metal resources and reduces costs and pollution compared to extracting virgin metals. However, recycling has costs and challenges like obtaining scrap metals.Ch10 study of compounds nitric acid
Ch10 study of compounds nitric acidRajiv Jain
Ėý
This document summarizes key questions and answers from a YouTube chemistry lesson on nitric acid and its reactions with metals and other compounds.
The lesson covers the preparation of nitric acid, its reactions with copper and sulfur, and the identification and equations for reactions of nitric acid with compounds like copper carbonate, lead nitrate, potassium nitrate, and ammonium nitrate. Key reactions include the decomposition of nitric acid and metal nitrates like copper nitrate and lead nitrate when heated. Equations are provided for the oxidation of sulfur and copper by concentrated nitric acid.Oxides
OxidessYhira
Ėý
This document discusses different types of oxides based on their reactions with water and acids or bases. It defines acidic, basic, amphoteric, and neutral oxides. Acidic oxides react with water to form acids, while basic oxides react with acids to form salts and water. Amphoteric oxides can behave as either acidic or basic oxides depending on the reaction. Neutral oxides show no acidic or basic properties and are insoluble in water. The document provides examples of common oxides that fall into each category and an decision tree for classifying an unknown oxide based on its solubility properties.igcse ORGANIC CHEMISTRY PRESENTATION.pptx
igcse ORGANIC CHEMISTRY PRESENTATION.pptxdongleiwhu
Ėý
This document provides an overview of organic chemistry, including:
- Organic chemistry is the study of carbon-containing compounds and their properties/reactions.
- Organic compounds contain carbon and can be classified as hydrocarbons, containing only carbon and hydrogen, or other organic functional groups.
- Key concepts include functional groups, homologous series, structural formulas and structural isomers. Reaction types like addition, substitution and elimination are also covered.Reaction of metals with water
Reaction of metals with water AakankshaYaduvanshi
Ėý
This document discusses the reactions of various metals with water, steam, and heat. Some reactive metals like sodium and potassium react vigorously with cold water, producing metal hydroxides and hydrogen gas. The reaction of potassium with cold water produces a colorless solution and burns with a lilac color. Calcium also reacts with cold water but does not ignite the hydrogen produced. Magnesium reacts with hot water but not cold water. Less reactive metals like aluminum, iron and zinc only react with steam, producing metal oxides and hydrogen gas. Some metals like lead, copper, silver and gold do not react at all with water, steam or heat.Acid Bases and Salts and Chemical Equations
Acid Bases and Salts and Chemical EquationsSanchit Duseja
Ėý
1) The document discusses chemical reactions and equations, focusing on chemical changes, types of chemical equations, and balanced chemical equations.
2) It then covers the five main types of chemical reactions - combination, decomposition, displacement, double displacement, and oxidation-reduction reactions.
3) Finally, it discusses acids, bases, and salts. It defines acids and bases, lists their key properties, methods of classification, common examples, and uses.Salt preparation
Salt preparation Zainab Yahya
Ėý
The document discusses four methods for preparing salts: 1) Reacting a metal with an acid, 2) Reacting an insoluble base with an acid, 3) Neutralizing an alkali with an acid through titration, and 4) Precipitation. It then provides examples of soluble and insoluble compounds, and explains how to specifically prepare zinc sulfate by reacting zinc powder with sulfuric acid. The document asks to describe how to prepare several example salts using these methods.6.3 (a) electrolysis of an aqueous solution
6.3 (a) electrolysis of an aqueous solutionAzieda Dot
Ėý
The document discusses the electrolysis of aqueous solutions. It explains that during electrolysis, only one ion is selectively discharged at each electrode based on its position in the electrochemical series, the nature of the electrode, and the concentration of ions. The ion discharged at the anode depends on which is easier to oxidize, while the ion discharged at the cathode depends on which is easier to reduce. Different products are formed depending on these factors and the specific electrolyte used.Y11 Chem - 2. Reactivity Series.pptx
Y11 Chem - 2. Reactivity Series.pptxKristieCorpus
Ėý
The document discusses the reactivity series of metals and how it is determined. The reactivity of a metal is based on its tendency to lose electrons and form positive ions. Experiments are conducted to see which metals react with water, acids, carbon, other metals, and metal ion solutions. These experiments allow metals to be ranked in a reactivity series from most reactive to least reactive. The reactivity series can then be used to predict and understand redox reactions between metals.Unit 6 Organic Chemistry
Unit 6 Organic ChemistryHaihao Liu
Ėý
Organic chemistry revision notes cover the formation of fossil fuels like oil from dead marine organisms under heat and pressure. Crude oil is separated into fractions like gasoline and kerosene through fractional distillation, and combustion produces pollution like carbon monoxide and nitrogen oxides. The energy released during combustion can be measured using a calorimeter. Homologous series are families of compounds with the same functional group and general formula that differ by CH2. Main series include alkanes, alkenes, and alcohols, which are named based on their carbon chain and functional group.Unit 2, Lesson 2.8 - Acids, Bases, and Salts
Unit 2, Lesson 2.8 - Acids, Bases, and Saltsjudan1970
Ėý
The document discusses the properties and differences between acids, bases, and salts, including their behavior in water, chemical formulas, and applications in the human body and industry. It also covers concepts such as the pH scale, neutralization reactions, and classifications of acids and bases. Additional educational activities and a quiz preparation outline are included at the end.Chemical reactions of acids and bases
Chemical reactions of acids and basesVeenuGupta8
Ėý
The document explains the properties and behaviors of acids and bases. Acids yield hydrogen ions (H+) in water and dissociate to form hydronium ions, whereas bases provide hydroxide ions (OHâ). It also discusses neutralization reactions, the heat released during the dissolution of acids and bases in water, and the reactions of acids with metals and carbonates.Acids, bases and salts IGCSE Chemistry
Acids, bases and salts IGCSE Chemistry Maitreyee Joshi
Ėý
This document provides an introduction to acids, bases, and salts for GCSE chemistry students. It discusses key topics like acidity and alkalinity, indicators, the pH scale, types of acids including strong and weak acids, and various methods for making salts through reactions between acids and metals, metal oxides, metal carbonates, metal hydroxides, and ammonia. The document is intended to help students understand and revise these core chemistry concepts in preparation for their exams.How to write a chemical formula
How to write a chemical formulaZahraFazal6
Ėý
This document discusses how to write chemical formulas. It explains that a chemical formula tells us the types and numbers of atoms in a compound. It then outlines five steps for writing chemical formulas: 1) Write the symbols of the ions side by side in order of positive then negative, 2) Write the valency of each ion, 3) Transfer the valencies to offset them, 4) Omit valencies that are the same, and include different valencies in the formula, 5) For radicals like sulfate and phosphate, the net charge indicates the radical's valency and it is written in parentheses.Similar to Ch8 study of compounds hydrogen chloride (18)
chlorine.pdf
chlorine.pdfBagalanaSteven
Ėý
Chlorine and its compounds are discussed. Chlorine can be prepared in the laboratory by reacting concentrated hydrochloric acid with manganese(IV) oxide or potassium manganate(VII). Chlorine is a greenish-yellow gas that is denser than air and acts as a strong oxidizing agent and bleach. It reacts with many metals and non-metals to form chlorides. Common uses of chlorine include water disinfection and manufacturing of bleaches, plastics and pesticides.Ch3 acid bases and salts
Ch3 acid bases and saltsRajiv Jain
Ėý
This document summarizes a YouTube chemistry video about acids, bases, and salts. It discusses the pH of salt solutions, methods of preparing salts, and properties of different salts like solubility and water of crystallization. It also contains sample chemistry questions and their answers about identifying oxides, salts, and writing chemical equations. Key topics covered include acid-base reactions, salt preparations, and properties of common salts and oxides.science- chemistry Study of Compounds.pptx
science- chemistry Study of Compounds.pptxSharmilaJayanthi1
Ėý
The document is a set of chemistry questions related to reactions involving hydrochloric acid and other compounds. It covers topics such as balanced equations, properties of gases, and specific chemical tests. Additionally, it addresses why certain substances should not be mixed and various uses of hydrochloric acid.CBSE CLASS 10 CHEMISTRY CHAPTER 1 CHEMICAL REACTIONS AND EQUATIONS PART 3 IN...
CBSE CLASS 10 CHEMISTRY CHAPTER 1 CHEMICAL REACTIONS AND EQUATIONS PART 3 IN...MeetaYadav5
Ėý
The document provides solutions to intext and back exercises from NCERT Class 10 Science Chapter 1 on chemical reactions and equations. It covers various aspects such as reasons for cleaning magnesium before burning, balancing chemical equations, identifying reaction types, and comparing oxidation and reduction processes. Additionally, it explains concepts of exothermic and endothermic reactions, precipitation reactions, and the significance of painting iron to prevent rusting.Revision on acid base and salt = with answers
Revision on acid base and salt = with answersMRSMPC
Ėý
This document provides information about chemistry revision on acids, bases and salts. It discusses soluble and insoluble salts such as chlorides, sulphates and nitrates. It also describes methods for preparing soluble and insoluble salts, including the titration and solid acid methods. The document further discusses the preparation of copper(II) sulphate through the reaction of copper(II) oxide with sulphuric acid, and provides chemical tests to identify the copper and sulphate ions.class-xChemical reaction corrosion and rancidity
class-xChemical reaction corrosion and rancidityTHE LEADERS ACADEMYKARAIKUDI
Ėý
The document provides an overview of various types of chemical reactions, including combination, decomposition, displacement, and double displacement reactions, with examples for each. It also discusses oxidation, reduction, corrosion, and rancidity, along with methods for preventing these processes. Additionally, it addresses the law of conservation of mass, characteristics of specific chemical reactions, and practical applications in everyday scenarios.Pre test2
Pre test2andromendas
Ėý
This document contains a chemistry exam for Form 4 students in Malaysia. It consists of 30 multiple choice questions testing concepts in chemistry including acids and bases, chemical reactions, and properties of substances. The questions cover topics such as acid-base reactions, properties of acids and bases, preparation of salts, pH and concentration, and identification of substances and chemical equations.Class X mOCK.docx
Class X mOCK.docxSubhendu Bandyopadhyay
Ėý
This document contains a mock chemistry test with multiple choice and short answer questions covering various topics:
1) The questions cover topics like duralumin alloys, methods of producing hydrogen chloride, reactions producing copper, addition reactions of bromine with alkenes, IUPAC and common names of organic compounds, isomers, production of chloroform and ethyne.
2) There are also questions about the chief ore of aluminum, its concentration process, electrolytic reduction, neutral oxides, catalysts, drying agents, balanced equations, properties of acids and the Ostwald process.
3) The final questions ask for IUPAC names and to identify muriatic acid and the main ore of iron.10th std HCl.pptx class 4 icse geography
10th std HCl.pptx class 4 icse geographyradhikadaksh1983
Ėý
The document outlines the laboratory preparation and properties of hydrogen chloride (HCl), including methods such as upward displacement of air for gas collection and various reactions showcasing its solubility and acidic nature. It details reactions with metals and bases, resulting in the formation of salts and gases, along with specific experiments demonstrating HCl's heavier-than-air property and its identification by white fumes with ammonia. Additionally, practical chemistry tests for HCl are discussed, including the formation of precipitates with silver nitrate and lead nitrate.chemo1-notes-studyguidepk.pdf
chemo1-notes-studyguidepk.pdfMathandScienced
Ėý
1. The document provides information on chemical formulae, equations, calculations involving moles, molar mass and volume. It also discusses common cationic and anionic symbols as well as formulae for several compounds.
2. Reaction details are given for group 1 and 17 elements with oxygen, halogens and water. The preparation of chlorine gas is also described.
3. Additional topics covered include electrolysis, acids and bases, properties of salts and effects of heating on different salts.CHAPTER 6 ACID, BASE AND SALT (1)
CHAPTER 6 ACID, BASE AND SALT (1)MISS ESTHER
Ėý
The document describes several experiments involving qualitative analysis of salts and acids. Salt X is identified as lead(II) carbonate from its reaction to produce solid Y and carbon dioxide gas when heated. Solution W is found to contain nitrate ions, identified through a test producing a brown ring with sulfuric acid and iron(II) sulfate. A precipitation reaction between solutions of lead nitrate and potassium iodide is used to calculate the mass of yellow precipitate formed. Strong acids such as hydrochloric acid and sulfuric acid are described as reacting with bases to produce salts and water, with metals to produce salts and hydrogen gas, and with carbonates to produce salts, carbon dioxide, and water. The role of water inChapter 4 Problems1. Which of these compounds is a strong elec.docx
Chapter 4 Problems1. Which of these compounds is a strong elec.docxketurahhazelhurst
Ėý
The document consists of a series of problems related to chemistry, focusing on topics such as electrolytes, oxidation numbers, solubility rules, chemical reactions (precipitation, acid-base neutralization, and combustion), molarity calculations, and stoichiometry. It includes multiple-choice questions and calculations involving chemical equations, molar mass, and percent yield. The content aims to test knowledge and understanding of fundamental chemical principles.Chapter 4 Problems1. Which of these compounds is a strong elec.docx
Chapter 4 Problems1. Which of these compounds is a strong elec.docxrobertad6
Ėý
The document consists of a series of chemistry problems covering topics such as electrolytes, oxidation-reduction reactions, solubility rules, and stoichiometry, with specific questions related to various compounds and reactions. It includes calculations for molarity, precipitation reactions, and balancing equations, as well as identifying BrÃļnsted acids and determining limiting reagents. Overall, the problems challenge the understanding of fundamental concepts in chemistry.Book 2
Book 2Max2026
Ėý
This document provides practice problems and solutions for chapters 14-24 of the HKDSE Chemistry textbook. It includes class practices and chapter exercises for each chapter, covering topics such as acids and bases, concentrations of solutions, indicators, strength of acids and bases, salts, hydrocarbons, and polymers. The problems aim to help students learn about important chemical concepts and calculations related to these topics.Ch sulphuric acid
Ch sulphuric acidRajiv Jain
Ėý
This document discusses sulphuric acid, including its production via the contact process using vanadium pentoxide as a catalyst, important properties such as being acidic, non-volatile, and an oxidizing agent. Sample reactions demonstrating these properties are given. The document also provides answers to questions about reactions of sulphuric acid such as its dehydrating effect on sugar, and how it can be used to distinguish between dilute hydrochloric acid and dilute sulphuric acid. Production of sulphuric acid and its widespread uses in industry and laboratories are covered.Ad
Ch8 study of compounds hydrogen chloride
- 1. YOU TUBE CHANNEL: KNOW THE CHEMISTRY BY RAJIV JAIN
- 2. THOUGHT FOR THE SESSION
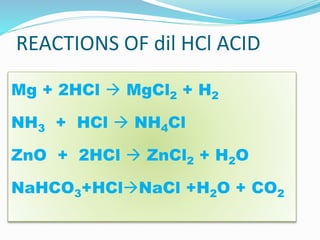
- 9. REACTIONS OF dil HCl ACID Mg + 2HCl ï MgCl2 + H2 NH3 + HCl ï NH4Cl ZnO + 2HCl ï ZnCl2 + H2O NaHCO3+HClï NaCl +H2O + CO2
- 10. REACTIONS OF dil HCl ACID KHSO3+ HCl ï KCl + H2O + SO2 ZnS + 2HCl ï ZnCl2 + H2S Na2S2O3 + 2HCl ï 2NaCl + H2O + SO2 + S Pb(NO3)2+ 2HCl ï PbCl2 â+ 2HNO3 AgNO3 + HCl ï AgCl â + HNO3
- 11. REACTIONS OF Conc. HCl
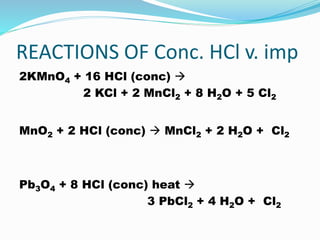
- 12. REACTIONS OF Conc. HCl v. imp 2KMnO4 + 16 HCl (conc) ï 2 KCl + 2 MnCl2 + 8 H2O + 5 Cl2 MnO2 + 2 HCl (conc) ï MnCl2 + 2 H2O + Cl2 Pb3O4 + 8 HCl (conc) heat ï 3 PbCl2 + 4 H2O + Cl2
- 13. Test for HCl ï BLUE Litmus RED / pH PAPER / INDICATORS (phenolpthalein/ methyl orange/ alkaline phenolpthalein) ï AgNO3 TEST / WHITE PPT ï AMMONIA SOLUTION TEST / DENSE WHITE FUMES OF AMONIUM CHLORIDE ï PUNGENT CHOCKING ODOUR ï COLOULESS GAS/ LIQUID IF HYDROCHLORIC ACID
- 14. IMPORTANT QUESTIONS ON HCl
- 15. Q1. This gas produces dense white fumes with ammonia gas. Ans1. Hydrogen chloride gas Q2. Identify the acid. âThe acid on mixing with silver nitrate solution produces a white precipitate which is soluble in excess ammonium hydroxideâ. Ans2. Hydrochloric acid
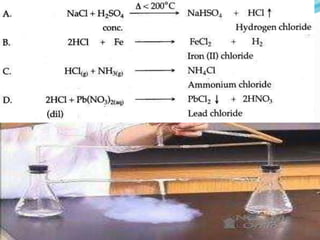
- 16. Q3. The following questions are pertaining to the laboratory preparation of hydrogen chloride gas. (i) Write the equation for its preparation mentioning the condition required. NaCl + H2SO4 Conc T< 2000C ï NaHSO4 + HCl (ii) Name the drying agent used and justify your choice. Conc H2SO4
- 17. Q3. The following questions are pertaining to the laboratory preparation of HCl (iii) State a safety precaution you would take during the preparation of hydrochloric acid. Temperature of the reaction mixture is less than 2000C
- 18. Q4. Fill in the blanks: (i) Quicklime is not used to dry HCl gas because __ (CaO is alkaline, CaO is acidic , CaO is neutral) alkaline
- 19. Q5. State one appropriate observation for each of the following: (i) Copper sulphide is treated with dilute hydrochloric acid. Colorless rotten egg gas evolved which turns moist lead acetate paper black. (ii) A few drops of dilute hydrochloric acid are added to silver nitrate solution, followed by addition of ammonia hydroxide solution. Curdy white ppt formed which dissolved in ammonium hydroxide solution.
- 20. Q6. Give balanced equations for the following reactions: (i)Concentrated hydrochloric acid and potassium permanganate solution Ans 2KMnO4 + 16 HCl conc ï 2 KCl + 2 MnCl2 + 8H2O + 6Cl2
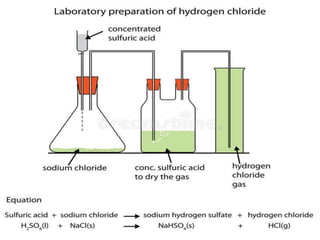
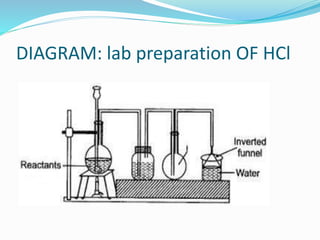
- 21. DIAGRAM: lab preparation OF HCl
- 22. Q7. In the laboratory preparation of hydrochloric acid, HCl gas is dissolved in water. (i) Draw a diagram to show the arrangement used for the absorption of HCl in water. (ii) Why is such an arrangement necessary? Give two reasons. Ans. Inverted funnel arrangement , To prevent the back suction . To make hydrochloric acid and to prevent any accident.
- 23. Q7. In the lab preparation of hydrochloric acid, HCl gas is dissolved in water. (iii) Write the chemical equations for the lab preparation of HCl gas when the reactants are: (A) Below 2000C (B) Above 2000C NaCl + H2SO4 Conc T< 2000C ï NaHSO4 + HCl 2NaCl + H2SO4 Conc T > 2000C ï Na2SO4 + 2HCl
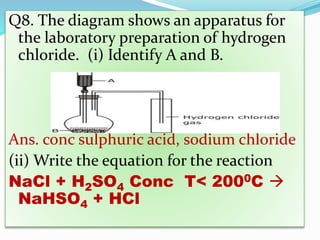
- 24. Q8. The diagram shows an apparatus for the laboratory preparation of hydrogen chloride. (i) Identify A and B. Ans. conc sulphuric acid, sodium chloride (ii) Write the equation for the reaction NaCl + H2SO4 Conc T< 2000C ï NaHSO4 + HCl
- 25. Q8. The diagram shows an apparatus for the lab preparation of hydrogen chloride. (iii) How would you check whether or not the gas jar is filled with hydrogen chloride? Ans. A rod dipped in ammonium hydroxide is brought near the mouth of the collecting jar. Dense white fumes signifies the gas is filled.
- 26. Q8. (iv) What does the method of collection tell you about the density of hydrogen chloride? Ans It is heavier than air.
- 27. Q9. Write a fully balanced equation for each of the following cases: (i)Red lead is warmed with concentrated hydrochloric acid. Ans. Pb3O4 + conc 8HCl ï 3PbCl2 + 4H2O + Cl2 (i) Magnesium metal is treated with dilute hydrochloric acid. Mg + 2HCl ï MgCl2 + H2
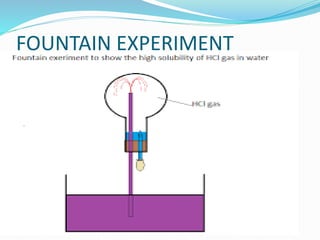
- 28. Q10. The diagram shows a simple arrangement of the fountain experiment: [3] (i) Name the two gases you have studied which can be used in this experiment. Ans, Ammonia, hydrogen chloride (i) What is the common property demonstrated by this experiment? High solubility of ammonia or hydrogen chloride in water.
- 29. Q11. (i) Of the two gases, ammonia and hydrogen chloride, which one is more dense? Name the method of collection of this gas. Ans. Hydrogen chloride , upward displacement of air. (ii) Give one example of a reaction between the above two gases, which produces a solid compound. NH3 + HCl ï NH4Cl
- 30. Q12. Write balanced equations for the reaction of dilute hydrochloric acid (i)Iron Fe + 2HCl ï FeCl2 +H2 (ii) Sodium hydrogen carbonate NaHCO3 + HCl ï NaCl + H2O + CO2 (iii) Iron (II) sulphide FeS + 2HCl ï FeCl2 +H2S
- 31. Q12. Write balanced equations for the reaction of dilute hydrochloric acid (iv) Sodium sulphite Na2SO3 + 2 HCl ï 2NaCl +H2O + SO2 (v) Sodium thiosulphate solution Na2S2O3 + 2 HCl ï 2NaCl +H2O + SO2 +S
- 32. Q13.A solution of hydrogen chloride in water is prepared. The following substance are added to separate portions of the solution: S. No. Substance added Gas evolved Odour 1 Calcium carbonate with dil HCl 2 Magnesium ribbon with dil HCl 3 Manganese (IV) oxide with conc HCl 4 Sodium sulphide with dil HCl
- 33. ANS13 S. No. Substance added Gas evolved Odour 1 Calcium carbonate with dil HCl Carbon dioxide Odourless 2 Magnesium ribbon with dil HCl Hydrogen Odourless 3 Manganese (IV) oxide with conc HCl Chlorine Pungent chocking 4 Sodium sulphide with dil HCl Hydrogen sulphide Rotten egg
- 34. Q14. What must be added to sodium chloride to get hydrogen chloride? Ans14. Conc sulphuric acid Q15. What would you see when hydrogen chloride mixes with ammonia? Ans15. Dense white fumes of ammonium chloride.
- 35. Q16. Write equation for the reaction of hydrochloric acid with (i) lead nitrate Pb(NO3)2 + 2HCl ï PbCl2 + 2HNO3 (ii) manganese (IV) oxide. MnO2 + 4HCl (conc) ï MnCl2 + 2H2O + Cl2
- 36. Q17. From the following list choose the correct answer: Ammonium hydroxide , ammonium nitrate, chlorine, dilute hydrochloric acid, iron, lead nitrate, manganese (IV) oxide, silver nitrate, sodium nitrate, sulphur. (i) A substance containing both molecules and ions. Ammonium hydroxide (ii) two compounds whose aqueous solution gives white precipitate with dilute hydrochloric acid. Ammonium hydroxide, silver nitrate solution
- 39. Please share and subscribe to gain knowledge from more videos âĶ. Facebook page and You tube channel: Know the Chemistry by Rajiv Jain
