Chemical formulae
Download as PPTX, PDF1 like1,150 views
Ionic compounds are composed of positive and negative ions. Positive ions, called cations, include sodium (Na+) and ammonium (NH4+). Negative ions, called anions, include chloride (Cl-) and carbonate (CO32-). Chemical formulas represent the elemental composition and ratios of ions that make up ionic compounds.
1 of 13
Downloaded 15 times












Recommended
Sodiumchloride 190723052155



Sodiumchloride 190723052155dipanshu chaurasiya
Ã˝
Sodium chloride, also known as salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 g/mol for sodium and 35.45 g/mol for chloride, 100 g of NaCl contains 39.34 g of sodium and 60.66 g of chloride.Stoichiometry: Chapter 9



Stoichiometry: Chapter 9vvchemistry
Ã˝
The document provides an introduction to stoichiometry including the four types of stoichiometry problems: (1) given and unknown amounts in moles, (2) given amount in moles and unknown in grams, (3) given amount in grams and unknown in moles, and (4) given and unknown amounts in grams. It then works through example problems of each type, calculating amounts of products or reactants using molar ratios derived from balanced chemical equations.Ionic equilibrium part 2



Ionic equilibrium part 2Damodar Koirala
Ã˝
The document summarizes key concepts regarding ionic equilibrium and solubility products. It discusses four types of salt hydrolysis and how they result in acidic, basic, or neutral solutions. It then defines solubility product and solubility product expressions for different types of salts. The solubility product principle is introduced, stating that precipitation will occur when the ionic product exceeds the solubility product constant.Ionic equilibrium part 1



Ionic equilibrium part 1Damodar Koirala
Ã˝
This document discusses ionic equilibrium and acid-base concepts. It defines strong and weak electrolytes, and explains that strong electrolytes completely ionize while weak electrolytes partially ionize. The degree of ionization and ionization constant are introduced to quantify the ionization of weak electrolytes. Factors that affect the degree of ionization are also discussed. Different theories of acids and bases are presented, including Arrhenius, Bronsted-Lowry, and Lewis concepts. The limitations of each theory and advantages of broader theories are described. Conjugated acid-base pairs are defined in relation to Bronsted acids and bases.Chapter 3 Chemical Formulae and Equations



Chapter 3 Chemical Formulae and EquationsM BR
Ã˝
This document discusses chemical formulae and equations. It defines relative atomic mass and relative molecular mass, which are used to calculate the mass of elements and compounds from their chemical formulae. The mole concept is introduced, relating the Avogadro constant to the number of particles in a given number of moles. Relationships are shown between moles, mass, particles and volume. Empirical and molecular formulae are distinguished. Ionic compounds have formulae showing cation and anion combinations. Examples of writing and balancing chemical equations are provided.Chemical Reactions With Acids



Chemical Reactions With AcidsAndrew Joseph
Ã˝
A tutorial covering, in the simple term, the how of neutralisation, acid metal reactions and acid carbonate reactions.#17 Key



#17 KeyLamar1411_SI
Ã˝
This document provides sample chemistry problems and questions related to topics like:
- Writing balanced equations for neutralization reactions
- Defining terms like oxidation, reduction, concentration, and indicators
- Determining oxidation states of elements in compounds
- Identifying redox, precipitation, and acid-base reactions
- Performing calculations involving molarity, moles, and concentration
The problems cover a wide range of general chemistry concepts.Azo compounds // compounds of Nitrogen



Azo compounds // compounds of NitrogenDr Shoaib Ahmad Bilal
Ã˝
Azo compounds contain the -N=N- azo group and are formed via a coupling reaction between a diazonium salt and a coupling agent. Diazonium salts are produced by diazotization, which involves reacting an arylamine with sodium nitrite and an acid below 5°C to form an unstable salt. This salt then reacts with a coupling agent containing an aromatic ring, producing a colored azo compound precipitate. Azo compounds are important dyes due to their stability and ability to produce different colors depending on the reactants used.AP Chemistry Chapter 4 Sample Exercise



AP Chemistry Chapter 4 Sample ExerciseJane Hamze
Ã˝
The document provides sample exercises to practice writing chemical equations and determining oxidation states. It includes questions about relating numbers of ions to chemical formulas, using solubility rules to classify compounds, predicting precipitation reactions, writing molecular and net ionic equations, identifying strong/weak electrolytes, and determining oxidation numbers of sulfur in various compounds. The exercises are accompanied by explanations of the thought processes and steps to arrive at the answers.9 Aqueous Solutions



9 Aqueous Solutionsjanetra
Ã˝
- Water is a polar solvent due to its molecular structure, with oxygen having a partial negative charge and hydrogen having partial positive charges. This allows it to dissolve ionic compounds by interacting with and separating the ions.
- Ionic compounds dissolve to varying degrees in water depending on how strongly the ions are attracted to each other versus water molecules. Solubility can be measured in g/L.
- Acids donate H+ ions in water and are classified as strong or weak based on how completely they ionize. Their strength affects pH calculations. Bases accept H+ and similarly ionize more or less completely.22 acids + bases



22 acids + basesmrtangextrahelp
Ã˝
This document discusses acids and bases according to several theories. It begins by describing the properties of acids, including reacting with metals and carbonates, conducting electricity, turning litmus paper colors, and neutralizing bases. It then discusses the properties of bases. The Arrhenius theory defines acids as substances that produce H+ ions in water and bases as those that produce OH- ions. However, this theory has limitations and does not account for all acids and bases. The Br√∏nsted-Lowry theory broadens the definition to any substance that can donate or accept protons. Strong acids fully dissociate in water while weak acids only partially dissociate. The pH scale measures the concentration of H+ ions on a#13 Key



#13 KeyLamar1411_SI
Ã˝
This document provides sample problems and questions for a general chemistry exam. It includes sample balanced equations for neutralization reactions and definitions of key terms like oxidation, reduction, concentration, and indicators. It also asks students to determine oxidation states, identify redox reactions, calculate molarity and amounts of substances in solutions, and determine concentrations of ions after mixing solutions.22 acids + bases



22 acids + basesmrtangextrahelp
Ã˝
The document discusses the key properties and reactions of acids and bases. It defines acids as substances that produce hydrogen ions (H+) in water and bases as substances that produce hydroxide ions (OH-). Acids react with metals, carbonates, conduct electricity, turn litmus paper red, and neutralize bases. Bases conduct electricity, turn litmus paper blue, and neutralize acids. Theories of acids and bases including Arrhenius, Br√∏nsted-Lowry, and Lewis are explained. Strong and weak acids/bases, monoprotic/diprotic/triprotic acids, pH, titrations, and acid-base indicators are also covered.Chapter 9 stoich



Chapter 9 stoichtanzmanj
Ã˝
Stoichiometry focuses on the mass and molar relationships in chemical reactions based on balanced equations. Key aspects include reading the balanced equation in terms of moles, determining all possible mole ratios such as moles to moles, moles to mass, mass to moles, and mass to mass, and using these relationships as a "recipe" to solve stoichiometry problems. The document provides examples of using a balanced equation to determine the moles of lithium hydroxide needed to react with a given amount of carbon dioxide and the grams of aluminum required to produce a certain amount of hydrogen gas.Chemical reactions and equations 



Chemical reactions and equations K. Shahzad Baig
Ã˝
Chemical reaction is the process by which a chemical change occurs.Equation reflects the true quantitative relationships Balancing a chemical equation.Stereochemistry



StereochemistrySirod Judin
Ã˝
This chapter discusses stereochemistry and chirality. It defines stereoisomers such as enantiomers, which are nonsuperimposable mirror images, and diastereomers, which are not mirror images. Chiral carbons have four different groups and exist as enantiomers. Enantiomers have identical properties except for how they interact with other chiral molecules and rotate plane-polarized light in opposite directions. Methods to determine chirality such as assigning R/S configurations and using Fischer projections are covered. The chapter also discusses resolving enantiomers through formation of diastereomers.90944 ass-2011



90944 ass-2011emmjay91
Ã˝
This document is an assessment schedule for a 2011 NCEA Level 1 Science test covering aspects of acids and bases. It provides the evidence statements and scoring criteria for four multi-part questions on the test. For each question, it describes the key points of information required for a response to be scored at the Achievement, Merit, or Excellence levels. Correct explanations relating subatomic particle arrangements and charges to ion formations are needed for top scores. The document also provides example answers and equations for acid-base reactions.Diazotisation and coupling reaction



Diazotisation and coupling reactionAyan saha
Ã˝
1. Diazotization is a reaction where an aryl amine like aniline reacts with nitrous acid to form an unstable diazonium ion intermediate.
2. Coupling reactions involve this diazonium ion reacting with compounds containing activating groups like phenol or aniline to form an azo product by joining the two aryl groups.
3. The coupling reaction is an electrophilic aromatic substitution that proceeds through a two-step mechanism where the diazonium ion attacks the aromatic ring in the first slow step.Redox 



Redox xinleho
Ã˝
Redox reactions involve the transfer of electrons between reactants, resulting in changes to their oxidation states. Oxidation is the loss of electrons or gain of oxygen, while reduction is the gain of electrons or loss of oxygen. The document provides examples of redox reactions and identifies the oxidizing and reducing agents and species undergoing oxidation and reduction. It also gives the oxidation states of elements in ammonium sulfate.Lecture8 carbohydrates



Lecture8 carbohydratessanisaddam
Ã˝
This document discusses carbohydrate classification and structures. It notes that carbohydrates are composed of carbon, hydrogen, and oxygen and are a major energy source. There are three types: monosaccharides, disaccharides, and polysaccharides. Monosaccharides can be classified as aldoses or ketoses depending on whether they contain an aldehyde or ketone group. Many monosaccharides form cyclic structures with 5-6 carbons through a reaction between a hydroxyl group and aldehyde/ketone. Common monosaccharides like glucose and fructose form specific cyclic structures called Haworth structures depending on whether they are in the alpha or beta configuration.#13



#13Lamar1411_SI
Ã˝
The document contains questions about chemistry concepts including:
1) Writing balanced molecular and net ionic equations for neutralization reactions between acids and bases.
2) Defining oxidation, reduction, concentration, molarity, and indicators.
3) Asking if oxidation can occur without accompanying reduction.
4) Calculating oxidation numbers of elements in compounds.
5) Identifying redox, precipitation, and acid-base reactions based on balanced equations.
6) Calculating molarity, moles of solute, and volumes of solutions.#18 Key



#18 KeyLamar1411_SI
Ã˝
1. When ions dissolve in water, they are surrounded by water molecules (hydrated).
2. Precipitation will occur when Na2CO3 and AgNO3 or FeSO4 and Pb(NO3)2 are mixed, but not NaNO3 and NiSO4.
3. Acids donate H+ ions, bases accept H+ ions. A monoprotic acid has one ionizable H, a diprotic acid has two. Strong acids fully ionize, weak acids only partially ionize.2nd Lecture on Ionic Equilibria | Chemistry Part I | 12th Std



2nd Lecture on Ionic Equilibria | Chemistry Part I | 12th StdAnsari Usama
Ã˝
1) This document discusses ionic equilibria, specifically the autoionization of water and the pH scale. It defines key terms like ionic product of water (Kw), pH, and pOH.
2) Salts can undergo hydrolysis when dissolved in water. Salts of strong acids and bases do not hydrolyze and are neutral. Salts of strong acids and weak bases hydrolyze to produce H3O+ ions, making the solution acidic. Salts of weak acids and strong bases hydrolyze to produce OH- ions, making the solution basic.
3) For salts of weak acids and bases, the solution can be acidic, basic, or neutral depending on whether the Ka3rd Lecture on Ionic Equilibria | Chemistry Part I | 12th Std



3rd Lecture on Ionic Equilibria | Chemistry Part I | 12th StdAnsari Usama
Ã˝
Buffer solutions resist changes in pH when small amounts of acid or base are added. There are two types: acidic buffers containing a weak acid and its salt, and basic buffers containing a weak base and its salt. The pH of a buffer solution is related to the ratio of the salt and acid or base concentrations. Buffer solutions have important applications in biological systems, agriculture, medicine, and analytical chemistry. They maintain pH despite additions of H+ or OH-.4. redox titrations



4. redox titrationsNikithaGopalpet
Ã˝
Learning objectives
Introduction
Preparation of a standard solution used for redox titration
Oxidizing and reducing agents used in volumetric analysis
N/10 potassium permanganate preparation
N/10 potassium dichromate preparation
N/10 Iodine solution preparation
Examples of redox titrations
Conclusion
References
Solutions and Chemical Reactions



Solutions and Chemical ReactionsBawa SmOky
Ã˝
This document discusses solutions and chemical reactions. It explains that water is important for life and chemistry as it can dissolve many ionic compounds and polar molecules. Solutions are homogeneous mixtures that can be characterized by their concentration in molarity units. Electrolytes, such as salts, acids, and bases, dissolve in water to form ions and conduct electricity to varying degrees. The concentrations of ions in solutions can be calculated from chemical equations. Chemical reactions in solutions, such as precipitation reactions, can be represented by molecular, complete ionic, and net ionic equations.#12 Key



#12 KeyLamar1411_SI
Ã˝
1. The document defines key chemistry terms including: aqueous solutions, solvents, solutes, electrolytes, nonelectrolytes, strong/weak electrolytes, and solvation.
2. Precipitation reactions are defined as reactions where an insoluble product called a precipitate forms. Molecular, complete ionic, spectator ions, and net ionic equations are also defined.
3. Strong acids and bases are listed as well as examples of soluble and insoluble compounds in water. Spectator ions are identified for sample precipitation reactions.12 types of chemical reactions



12 types of chemical reactionsmrtangextrahelp
Ã˝
This document defines and provides examples of different types of chemical reactions including synthesis, decomposition, single displacement, double displacement, neutralization, and combustion reactions. Key aspects that determine the reaction type are whether reactants are elements, compounds, or if oxygen is involved. The position of elements in the periodic table also provides clues about reactivity in displacement reactions.molecules and compounds-structure and reactivity



molecules and compounds-structure and reactivityAkefAfaneh2
Ã˝
molecules and compounds-structure and reactivityUnit 7 chemical names & formulas



Unit 7 chemical names & formulastreothe
Ã˝
1) The document discusses predicting ionic charges and writing formulas for ionic compounds.
2) It provides examples of determining the empirical and molecular formulas for different compounds.
3) The key steps shown are calculating formula mass, percentage composition, and determining empirical formulas based on the mass percentages of elements in a compound.More Related Content
What's hot (20)
AP Chemistry Chapter 4 Sample Exercise



AP Chemistry Chapter 4 Sample ExerciseJane Hamze
Ã˝
The document provides sample exercises to practice writing chemical equations and determining oxidation states. It includes questions about relating numbers of ions to chemical formulas, using solubility rules to classify compounds, predicting precipitation reactions, writing molecular and net ionic equations, identifying strong/weak electrolytes, and determining oxidation numbers of sulfur in various compounds. The exercises are accompanied by explanations of the thought processes and steps to arrive at the answers.9 Aqueous Solutions



9 Aqueous Solutionsjanetra
Ã˝
- Water is a polar solvent due to its molecular structure, with oxygen having a partial negative charge and hydrogen having partial positive charges. This allows it to dissolve ionic compounds by interacting with and separating the ions.
- Ionic compounds dissolve to varying degrees in water depending on how strongly the ions are attracted to each other versus water molecules. Solubility can be measured in g/L.
- Acids donate H+ ions in water and are classified as strong or weak based on how completely they ionize. Their strength affects pH calculations. Bases accept H+ and similarly ionize more or less completely.22 acids + bases



22 acids + basesmrtangextrahelp
Ã˝
This document discusses acids and bases according to several theories. It begins by describing the properties of acids, including reacting with metals and carbonates, conducting electricity, turning litmus paper colors, and neutralizing bases. It then discusses the properties of bases. The Arrhenius theory defines acids as substances that produce H+ ions in water and bases as those that produce OH- ions. However, this theory has limitations and does not account for all acids and bases. The Br√∏nsted-Lowry theory broadens the definition to any substance that can donate or accept protons. Strong acids fully dissociate in water while weak acids only partially dissociate. The pH scale measures the concentration of H+ ions on a#13 Key



#13 KeyLamar1411_SI
Ã˝
This document provides sample problems and questions for a general chemistry exam. It includes sample balanced equations for neutralization reactions and definitions of key terms like oxidation, reduction, concentration, and indicators. It also asks students to determine oxidation states, identify redox reactions, calculate molarity and amounts of substances in solutions, and determine concentrations of ions after mixing solutions.22 acids + bases



22 acids + basesmrtangextrahelp
Ã˝
The document discusses the key properties and reactions of acids and bases. It defines acids as substances that produce hydrogen ions (H+) in water and bases as substances that produce hydroxide ions (OH-). Acids react with metals, carbonates, conduct electricity, turn litmus paper red, and neutralize bases. Bases conduct electricity, turn litmus paper blue, and neutralize acids. Theories of acids and bases including Arrhenius, Br√∏nsted-Lowry, and Lewis are explained. Strong and weak acids/bases, monoprotic/diprotic/triprotic acids, pH, titrations, and acid-base indicators are also covered.Chapter 9 stoich



Chapter 9 stoichtanzmanj
Ã˝
Stoichiometry focuses on the mass and molar relationships in chemical reactions based on balanced equations. Key aspects include reading the balanced equation in terms of moles, determining all possible mole ratios such as moles to moles, moles to mass, mass to moles, and mass to mass, and using these relationships as a "recipe" to solve stoichiometry problems. The document provides examples of using a balanced equation to determine the moles of lithium hydroxide needed to react with a given amount of carbon dioxide and the grams of aluminum required to produce a certain amount of hydrogen gas.Chemical reactions and equations 



Chemical reactions and equations K. Shahzad Baig
Ã˝
Chemical reaction is the process by which a chemical change occurs.Equation reflects the true quantitative relationships Balancing a chemical equation.Stereochemistry



StereochemistrySirod Judin
Ã˝
This chapter discusses stereochemistry and chirality. It defines stereoisomers such as enantiomers, which are nonsuperimposable mirror images, and diastereomers, which are not mirror images. Chiral carbons have four different groups and exist as enantiomers. Enantiomers have identical properties except for how they interact with other chiral molecules and rotate plane-polarized light in opposite directions. Methods to determine chirality such as assigning R/S configurations and using Fischer projections are covered. The chapter also discusses resolving enantiomers through formation of diastereomers.90944 ass-2011



90944 ass-2011emmjay91
Ã˝
This document is an assessment schedule for a 2011 NCEA Level 1 Science test covering aspects of acids and bases. It provides the evidence statements and scoring criteria for four multi-part questions on the test. For each question, it describes the key points of information required for a response to be scored at the Achievement, Merit, or Excellence levels. Correct explanations relating subatomic particle arrangements and charges to ion formations are needed for top scores. The document also provides example answers and equations for acid-base reactions.Diazotisation and coupling reaction



Diazotisation and coupling reactionAyan saha
Ã˝
1. Diazotization is a reaction where an aryl amine like aniline reacts with nitrous acid to form an unstable diazonium ion intermediate.
2. Coupling reactions involve this diazonium ion reacting with compounds containing activating groups like phenol or aniline to form an azo product by joining the two aryl groups.
3. The coupling reaction is an electrophilic aromatic substitution that proceeds through a two-step mechanism where the diazonium ion attacks the aromatic ring in the first slow step.Redox 



Redox xinleho
Ã˝
Redox reactions involve the transfer of electrons between reactants, resulting in changes to their oxidation states. Oxidation is the loss of electrons or gain of oxygen, while reduction is the gain of electrons or loss of oxygen. The document provides examples of redox reactions and identifies the oxidizing and reducing agents and species undergoing oxidation and reduction. It also gives the oxidation states of elements in ammonium sulfate.Lecture8 carbohydrates



Lecture8 carbohydratessanisaddam
Ã˝
This document discusses carbohydrate classification and structures. It notes that carbohydrates are composed of carbon, hydrogen, and oxygen and are a major energy source. There are three types: monosaccharides, disaccharides, and polysaccharides. Monosaccharides can be classified as aldoses or ketoses depending on whether they contain an aldehyde or ketone group. Many monosaccharides form cyclic structures with 5-6 carbons through a reaction between a hydroxyl group and aldehyde/ketone. Common monosaccharides like glucose and fructose form specific cyclic structures called Haworth structures depending on whether they are in the alpha or beta configuration.#13



#13Lamar1411_SI
Ã˝
The document contains questions about chemistry concepts including:
1) Writing balanced molecular and net ionic equations for neutralization reactions between acids and bases.
2) Defining oxidation, reduction, concentration, molarity, and indicators.
3) Asking if oxidation can occur without accompanying reduction.
4) Calculating oxidation numbers of elements in compounds.
5) Identifying redox, precipitation, and acid-base reactions based on balanced equations.
6) Calculating molarity, moles of solute, and volumes of solutions.#18 Key



#18 KeyLamar1411_SI
Ã˝
1. When ions dissolve in water, they are surrounded by water molecules (hydrated).
2. Precipitation will occur when Na2CO3 and AgNO3 or FeSO4 and Pb(NO3)2 are mixed, but not NaNO3 and NiSO4.
3. Acids donate H+ ions, bases accept H+ ions. A monoprotic acid has one ionizable H, a diprotic acid has two. Strong acids fully ionize, weak acids only partially ionize.2nd Lecture on Ionic Equilibria | Chemistry Part I | 12th Std



2nd Lecture on Ionic Equilibria | Chemistry Part I | 12th StdAnsari Usama
Ã˝
1) This document discusses ionic equilibria, specifically the autoionization of water and the pH scale. It defines key terms like ionic product of water (Kw), pH, and pOH.
2) Salts can undergo hydrolysis when dissolved in water. Salts of strong acids and bases do not hydrolyze and are neutral. Salts of strong acids and weak bases hydrolyze to produce H3O+ ions, making the solution acidic. Salts of weak acids and strong bases hydrolyze to produce OH- ions, making the solution basic.
3) For salts of weak acids and bases, the solution can be acidic, basic, or neutral depending on whether the Ka3rd Lecture on Ionic Equilibria | Chemistry Part I | 12th Std



3rd Lecture on Ionic Equilibria | Chemistry Part I | 12th StdAnsari Usama
Ã˝
Buffer solutions resist changes in pH when small amounts of acid or base are added. There are two types: acidic buffers containing a weak acid and its salt, and basic buffers containing a weak base and its salt. The pH of a buffer solution is related to the ratio of the salt and acid or base concentrations. Buffer solutions have important applications in biological systems, agriculture, medicine, and analytical chemistry. They maintain pH despite additions of H+ or OH-.4. redox titrations



4. redox titrationsNikithaGopalpet
Ã˝
Learning objectives
Introduction
Preparation of a standard solution used for redox titration
Oxidizing and reducing agents used in volumetric analysis
N/10 potassium permanganate preparation
N/10 potassium dichromate preparation
N/10 Iodine solution preparation
Examples of redox titrations
Conclusion
References
Solutions and Chemical Reactions



Solutions and Chemical ReactionsBawa SmOky
Ã˝
This document discusses solutions and chemical reactions. It explains that water is important for life and chemistry as it can dissolve many ionic compounds and polar molecules. Solutions are homogeneous mixtures that can be characterized by their concentration in molarity units. Electrolytes, such as salts, acids, and bases, dissolve in water to form ions and conduct electricity to varying degrees. The concentrations of ions in solutions can be calculated from chemical equations. Chemical reactions in solutions, such as precipitation reactions, can be represented by molecular, complete ionic, and net ionic equations.#12 Key



#12 KeyLamar1411_SI
Ã˝
1. The document defines key chemistry terms including: aqueous solutions, solvents, solutes, electrolytes, nonelectrolytes, strong/weak electrolytes, and solvation.
2. Precipitation reactions are defined as reactions where an insoluble product called a precipitate forms. Molecular, complete ionic, spectator ions, and net ionic equations are also defined.
3. Strong acids and bases are listed as well as examples of soluble and insoluble compounds in water. Spectator ions are identified for sample precipitation reactions.12 types of chemical reactions



12 types of chemical reactionsmrtangextrahelp
Ã˝
This document defines and provides examples of different types of chemical reactions including synthesis, decomposition, single displacement, double displacement, neutralization, and combustion reactions. Key aspects that determine the reaction type are whether reactants are elements, compounds, or if oxygen is involved. The position of elements in the periodic table also provides clues about reactivity in displacement reactions.Similar to Chemical formulae (20)
molecules and compounds-structure and reactivity



molecules and compounds-structure and reactivityAkefAfaneh2
Ã˝
molecules and compounds-structure and reactivityUnit 7 chemical names & formulas



Unit 7 chemical names & formulastreothe
Ã˝
1) The document discusses predicting ionic charges and writing formulas for ionic compounds.
2) It provides examples of determining the empirical and molecular formulas for different compounds.
3) The key steps shown are calculating formula mass, percentage composition, and determining empirical formulas based on the mass percentages of elements in a compound.Lecture 7.2- Ionic Compounds



Lecture 7.2- Ionic CompoundsMary Beth Smith
Ã˝
The document discusses ionic compounds and their properties. It defines ionic compounds as compounds composed of cations and anions held together by ionic bonds. Ionic compounds form crystalline structures with ions arranged in a repeating pattern. They are typically solids at room temperature due to their high melting points from the strong electrostatic forces between ions.Hydrocarbon class 11th .pptx



Hydrocarbon class 11th .pptxLakshay Singh
Ã˝
The document provides information about conformations in hydrocarbons. It discusses that carbon-carbon single bonds allow rotation, leading to different conformations. Ethane is used as an example to explain staggered and eclipsed conformations. The relative stabilities of these conformations are also mentioned. Further, the document covers alkenes, alkynes and aromatic hydrocarbons. It provides their structures, properties and reactions like addition, oxidation, halogenation etc.Chapter1



Chapter1Adnan Sohail
Ã˝
This document provides an overview of different chapters of fundamentals of chemistry. It defines key terms like biochemistry, industrial chemistry, nuclear chemistry and organic chemistry. It also discusses classification of substances as elements, compounds and mixtures. Examples are provided to differentiate between homogeneous and heterogeneous mixtures. Concepts like empirical formula, molecular formula, atomic mass unit and mole are explained. Mass to mole conversions are demonstrated through examples.11th_chemistry_unit_1_ppt_em_218218.pptx



11th_chemistry_unit_1_ppt_em_218218.pptxLUXMIKANTGIRI
Ã˝
This document provides an overview of basic chemistry concepts and calculations. It begins by explaining that chemistry is involved in everyday life through materials like polymers, drugs, and alloys. Matter can be classified based on physical state as solid, liquid, or gas, and chemically as pure substances or mixtures. Elements are composed of single atom types that can be monatomic or polyatomic. Compounds contain two or more elements. The mole concept is introduced to quantify atoms and molecules using Avogadro's number. Empirical and molecular formulas are distinguished. Methods for determining formulas from elemental analysis or molar mass are presented. Equivalent mass and stoichiometric calculations are also covered.namma_kalvi_11th_chemistry_unit_1_ppt_em_218218.pptx



namma_kalvi_11th_chemistry_unit_1_ppt_em_218218.pptxLUXMIKANTGIRI
Ã˝
This document provides an overview of basic chemistry concepts and calculations. It begins by explaining that chemistry is involved in everyday life through materials like polymers, drugs, and alloys. Matter can be classified based on physical state as solid, liquid, or gas, and chemically as pure substances or mixtures. Elements are composed of single atom types that can be monatomic or polyatomic. Compounds contain two or more elements. The mole concept is introduced to quantify atoms and molecules using Avogadro's number of 6.022x1023 particles. Empirical and molecular formulas are distinguished. Methods are described for determining empirical formulas from elemental analysis data and calculating molecular formulas. Stoichiometry allows determining quantitative relationships between reactants and products in chemical equations9th Chemistry Notes - Test Yourself (Malik Xufyan)



9th Chemistry Notes - Test Yourself (Malik Xufyan)Malik Xufyan
Ã˝
This document contains practice questions and answers related to 9th grade chemistry notes. It is divided into multiple sections covering topics like branches of chemistry, physical and chemical properties of substances, atomic structure, moles and molar masses. The questions are in a MCQ format testing the understanding of fundamental concepts. For example, one question asks the student to identify the branch of chemistry that deals with atomic energy and its uses in daily life. The answer given is nuclear chemistry._kalvi_11t.pptx



_kalvi_11t.pptxjuha21
Ã˝
This document provides an overview of basic chemistry concepts and calculations. It begins by explaining that chemistry is involved in everyday life through materials like polymers, drugs, and alloys. Matter can be classified based on physical state as solid, liquid, or gas, and chemically as pure substances or mixtures. Elements are composed of single atom types, while compounds contain two or more different atom types bonded together. The mole concept allows chemists to quantify amounts of substances down to the molecular level. Chemical calculations can be performed based on molar mass, stoichiometry from balanced equations, and other relationships between amounts of reactants and products.Chapter 3



Chapter 3Lama K Banna
Ã˝
First Year in Dentistry First Semester
Al-Azhar University - Gaza
Lama El Banna
general chemistry CALCULATIONS WITH CHEMICAL FORMULAS AND EQUATION



CALCULATIONS WITH CHEMICAL FORMULAS AND EQUATIONINSTITUTO TECNOLÓGICO DE SONORA
Ã˝
Calculate the empirical formula of vitamin C from the
given data.Carbenes



Carbenesbittukumar110
Ã˝
1. Carbenes are neutral molecules containing a divalent carbon atom with six electrons and two substituents. They exist as either singlet or triplet states depending on the electronic spin.
2. Carbenes can be generated through reactions such as α-elimination of halogenated compounds with bases, thermal decomposition of diazo compounds, and metal-catalyzed decomposition of diazo carbonyl compounds.
3. Carbenes undergo several types of reactions including insertion into bonds, addition to multiple bonds, and rearrangements. Notable reactions include C-H and N-H insertions, cyclopropanation of alkenes, and Wolff rearrangement to form ketenes.Carbenes .......



Carbenes .......keerthanan77
Ã˝
1. Carbenes are neutral molecules containing a divalent carbon atom with two unshared valence electrons. They exist in both singlet and triplet states depending on the electronic spin.
2. Carbenes undergo insertion reactions into X-H and C-C bonds. They also add across double bonds, with singlet carbenes preserving alkene stereochemistry and triplet carbenes losing it.
3. Carbenes are generated by reactions such as α-elimination of halogenated compounds with base or decomposition of diazo compounds. They can rearrange through migrations such as the Wolff or Arndt-Eistert reactions.Chem 101 week 4



Chem 101 week 4tdean1
Ã˝
Chem 101 Powerpoints Week ending 2/10/2012
Chapter 3 Part 1 covers naming binary ionic compounds containing metals and non-metals, writing formulas from names, and mass relationships in chemical reactions including molar mass, moles, and percent composition. Key topics include naming compounds based on the type of metal cation present (Type I vs Type II), rules for naming acids and polyatomic ions, calculating formula mass from chemical formulas, using the molar mass to determine the number of particles in a given mass of a substance, and calculating the percent composition of elements in compounds.CHEM-LEC-LESSON-4.pdf



CHEM-LEC-LESSON-4.pdfAce Dumpp
Ã˝
1) Corrosion is the reaction of a metal with its environment that causes it to convert to a metal compound. This occurs as the metal loses electrons and forms cations that combine with anions.
2) Redox reactions involve the transfer of electrons from one substance to another, causing a change in oxidation states. Reduction occurs when an atom gains electrons and is reduced, while oxidation occurs when an atom loses electrons and is oxidized.
3) Ions are formed when atoms gain or lose electrons, becoming cations if positively charged or anions if negatively charged. Oxidation numbers indicate the charge of an atom in a compound.Science 10th Class 



Science 10th Class Rahul Thakur
Ã˝
This document provides information on stoichiometry, which involves using mole ratios from balanced chemical equations to calculate mass relationships between substances in a chemical reaction. It outlines the steps to solve stoichiometry problems, which include writing a balanced equation, identifying known and unknown quantities, setting up mole ratio conversion factors between moles of reactants and products, and checking the answer. Key concepts discussed include the mole ratio from coefficients in a balanced equation, molar mass to convert between moles and grams, and the molar volume used to calculate liters of gas at standard temperature and pressure.Molar Mass



Molar Massguest503ed1
Ã˝
This document discusses predicting formulas for ionic and covalent compounds using oxidation numbers and prefixes. It provides an example of calculating the molar mass of an ionic compound, sodium oxide (Na2O), with a molar mass of 62g, found by looking up the atomic masses of sodium (23g/mol) and oxygen (16g/mol) and adding their contributions based on the formula. It also gives the example of carbon tetrachloride (CCl4) as a covalent compound with a molar mass of 152g based on the atomic masses of carbon (12g) and chlorine (35g).Solubility Products



Solubility Productswalt sautter
Ã˝
Discusses the chemical of slightly soluble compounds. Ksp and factors affecting solubility are included as well as solved problems.
**More good stuff available at:
www.wsautter.com
and
http://www.youtube.com/results?search_query=wnsautter&aq=fRecently uploaded (20)
How to Configure Deliver Content by Email in Odoo 18 Sales



How to Configure Deliver Content by Email in Odoo 18 SalesCeline George
Ã˝
In this slide, we’ll discuss on how to configure proforma invoice in Odoo 18 Sales module. A proforma invoice is a preliminary invoice that serves as a commercial document issued by a seller to a buyer.Intellectual Honesty & Research Integrity.pptx



Intellectual Honesty & Research Integrity.pptxNidhiSharma495177
Ã˝
Research Publication & Ethics contains a chapter on Intellectual Honesty and Research Integrity.
Different case studies of intellectual dishonesty and integrity were discussed.How to Configure Recurring Revenue in Odoo 17 CRM



How to Configure Recurring Revenue in Odoo 17 CRMCeline George
Ã˝
This slide will represent how to configure Recurring revenue. Recurring revenue are the income generated at a particular interval. Typically, the interval can be monthly, yearly, or we can customize the intervals for a product or service based on its subscription or contract. Functional Muscle Testing of Facial Muscles.pdf



Functional Muscle Testing of Facial Muscles.pdfSamarHosni3
Ã˝
Functional Muscle Testing of Facial Muscles.pdfHelping Autistic Girls Shine Webinar ∫›∫›fl£s



Helping Autistic Girls Shine Webinar ∫›∫›fl£sPooky Knightsmith
Ã˝
For more information about my speaking and training work, visit: https://www.pookyknightsmith.com/speaking/Interim Guidelines for PMES-DM-17-2025-PPT.pptx



Interim Guidelines for PMES-DM-17-2025-PPT.pptxsirjeromemanansala
Ã˝
This is the latest issuance on PMES as replacement of RPMS. Kindly message me to gain full access of the presentation. AI and Academic Writing, Short Term Course in Academic Writing and Publicatio...



AI and Academic Writing, Short Term Course in Academic Writing and Publicatio...Prof. (Dr.) Vinod Kumar Kanvaria
Ã˝
AI and Academic Writing, Short Term Course in Academic Writing and Publication, UGC-MMTTC, MANUU, 25/02/2025, Prof. (Dr.) Vinod Kumar Kanvaria, University of Delhi, vinodpr111@gmail.comOral exam Kenneth Bech - What is the meaning of strategic fit?



Oral exam Kenneth Bech - What is the meaning of strategic fit?MIPLM
Ã˝
Presentation of the CEIPI DU IPBA oral exam of Kenneth Bech - What is the meaning of strategic fit? ASP.NET Interview Questions PDF By ScholarHat



ASP.NET Interview Questions PDF By ScholarHatScholarhat
Ã˝
ASP.NET Interview Questions PDF By ScholarHatAdministrative bodies( D and C Act, 1940



Administrative bodies( D and C Act, 1940P.N.DESHMUKH
Ã˝
These presentation include information about administrative bodies such as D.T.A.B
CDL AND DCC, etc.ASP.NET Web API Interview Questions By Scholarhat



ASP.NET Web API Interview Questions By ScholarhatScholarhat
Ã˝
ASP.NET Web API Interview Questions By ScholarhatUnit 1 Computer Hardware for Educational Computing.pptx



Unit 1 Computer Hardware for Educational Computing.pptxRomaSmart1
Ã˝
Computers have revolutionized various sectors, including education, by enhancing learning experiences and making information more accessible. This presentation, "Computer Hardware for Educational Computing," introduces the fundamental aspects of computers, including their definition, characteristics, classification, and significance in the educational domain. Understanding these concepts helps educators and students leverage technology for more effective learning.Entity Framework Interview Questions PDF By ScholarHat



Entity Framework Interview Questions PDF By ScholarHatScholarhat
Ã˝
Entity Framework Interview Questions PDF By ScholarHatBỘ TEST KIỂM TRA GIỮA KÌ 2 - TIẾNG ANH 10,11,12 - CHUẨN FORM 2025 - GLOBAL SU...



BỘ TEST KIỂM TRA GIỮA KÌ 2 - TIẾNG ANH 10,11,12 - CHUẨN FORM 2025 - GLOBAL SU...Nguyen Thanh Tu Collection
Ã˝
https://app.box.com/s/ij1ty3vm7el9i4qfrr41o756xycbahmgMastering Soft Tissue Therapy & Sports Taping



Mastering Soft Tissue Therapy & Sports TapingKusal Goonewardena
Ã˝
Mastering Soft Tissue Therapy & Sports Taping: Pathway to Sports Medicine Excellence
This presentation was delivered in Colombo, Sri Lanka, at the Institute of Sports Medicine to an audience of sports physiotherapists, exercise scientists, athletic trainers, and healthcare professionals. Led by Kusal Goonewardena (PhD Candidate - Muscle Fatigue, APA Titled Sports & Exercise Physiotherapist) and Gayath Jayasinghe (Sports Scientist), the session provided comprehensive training on soft tissue assessment, treatment techniques, and essential sports taping methods.
Key topics covered:
✅ Soft Tissue Therapy – The science behind muscle, fascia, and joint assessment for optimal treatment outcomes.
✅ Sports Taping Techniques – Practical applications for injury prevention and rehabilitation, including ankle, knee, shoulder, thoracic, and cervical spine taping.
✅ Sports Trainer Level 1 Course by Sports Medicine Australia – A gateway to professional development, career opportunities, and working in Australia.
This training mirrors the Elite Akademy Sports Medicine standards, ensuring evidence-based approaches to injury management and athlete care.
If you are a sports professional looking to enhance your clinical skills and open doors to global opportunities, this presentation is for you.AI and Academic Writing, Short Term Course in Academic Writing and Publicatio...



AI and Academic Writing, Short Term Course in Academic Writing and Publicatio...Prof. (Dr.) Vinod Kumar Kanvaria
Ã˝
BỘ TEST KIỂM TRA GIỮA KÌ 2 - TIẾNG ANH 10,11,12 - CHUẨN FORM 2025 - GLOBAL SU...



BỘ TEST KIỂM TRA GIỮA KÌ 2 - TIẾNG ANH 10,11,12 - CHUẨN FORM 2025 - GLOBAL SU...Nguyen Thanh Tu Collection
Ã˝
Chemical formulae
- 1. CHEMICAL FORMULAE – MOLECULAR FORMULA • Ionic compounds are compounds made up of ions. • Ions are atoms or groups of atoms with positive or negative charges on them. • Positive ions are called cations. Examples include the sodium ion, Na+ and the ammonium ion, NH4 +. • Negative ions are called anions. Examples include the chloride ion, CI- , and the carbonate ion, CO3 2 -.
- 11. CHEMICAL EQUATIONS - SOLUTIONS
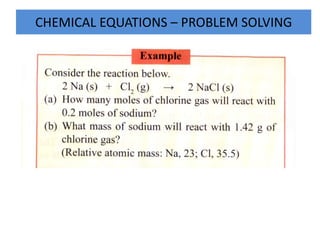
- 12. CHEMICAL EQUATIONS – PROBLEM SOLVING
- 13. CHEMICAL EQUATIONS – PROBLEM SOLVING Solution: (a) 2 moles of Na react with 1 mole of CI2. 1 mole of Na will react with ⅟2 moles of Cl2 Hence 0.2 moles of Na will react with ⅟2 x 0.2 = 0.1 mole of Cl2. (b) Molar mass of Cl2 = 2 x 35.5 = 71 g mol- 1 Number of moles of Cl2=1.42/71 = 0.02 moles 1 mole of Cl2 reacts with 2 moles of Na, 0.02 moles of CI2 will react with 0.02 x 2 = 0.04 mole of Na. Therefore, mass of Na = 0.04 x Ar of Na = 0.04 x 23 = 0.92 g.







