Christel Nourissier Cavod 06 09
- 1. www.eurordis.org EU INITIATIVES TO IMPROVE ACCESS TO CARE FOR RARE DISEASE PATIENTS Christel Nourissier General Secretary, EURORDIS Balkan Congress Rare Diseases_26-27 June 2009
- 2. www.eurordis.org A new EU Legislative and Policy Framework For Treatment of Rare Diseases
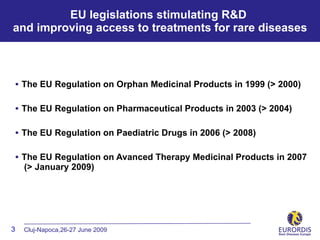
- 3. EU legislations stimulating R&D and improving access to treatments for rare diseases ÔÇó The EU Regulation on Orphan Medicinal Products in 1999 (> 2000) ÔÇó The EU Regulation on Pharmaceutical Products in 2003 (> 2004) ÔÇó The EU Regulation on Paediatric Drugs in 2006 (> 2008) ÔÇó The EU Regulation on Avanced Therapy Medicinal Products in 2007 (> January 2009) 3 Cluj-Napoca,26-27 June 2009
- 4. Member States have EU common procedures for: ÔÇó ODD= Orphan Drug Designation: Committee for Orphan Medicinal Products, COMP/EMEA ÔÇó PA= Protocol Assistance: Scientific Advice Working Party, SAWP/CHMP/COMP/EMEA ÔÇó PIP = Paediatric Investigation Plan: Paediatrics Committee, PDCO/ EMEA ÔÇó CAT = Committee for Advanced Therapies ÔÇó MAA = Marketing Authorisation Application: Committee for Medicinal Products for Human Use, CHMP/EMEA - Revision of orphan designation criteria at MAA (including Significant benefit): COMP/EMEA ÔÇó Post-MA obligations, additional studies, follow-up registries assessed by CHMP/EMEA 4 Cluj-Napoca,26-27 June 2009
- 5. Availability from the patientsÔÇÖ point of view Designation, Marketing Authorisation : European process EMEA EU National availability for patients ? 5 Cluj-Napoca,26-27 June 2009
- 6. A new policy base to improve availability ÔÇó The EU Pharmaceutical Forum Conclusions & Recommendations (adopted November 2008) : ´éº Guiding Principles on ┬½ Improving Access to orphan medicines for all affected EU citizens ┬╗ ´éº Guiding Principles on Relative Effectiveness ÔÇó The Commission Communication on Rare Diseases, Chapter 5.3 ┬½ Access to Orphan Drugs ┬╗ (adopted december 2008) ÔÇó The Council Recommendation on Rare Diseases, Chapter 5 ┬½ Gathering the expertise on rare diseases at the European level ┬╗ 5.5 ┬½ therapeutic or clinical added value of orpahn drugs ┬╗ (adopted June 2009) 6 Cluj-Napoca,26-27 June 2009
- 7. Key Facts & Values relevant to availability ÔÇó Rarity of patients ÔÇó Scarcity of expertise & knowledge ÔÇó Overeaching values of universality, access to good quality care, equity, solidarity, social justice 7 Cluj-Napoca,26-27 June 2009
- 8. www.eurordis.org Inequalities of patientÔÇÖs access to treatments And national challenges to be addressed
- 9. Inequalities of access: a EuropeÔÇÖs Challenge ÔÇó EURORDIS Surveys on Availability of all Orphan Medicines across all EU Member States, performed in 2001, 2003, 2005, 2007 ÔÇó Major inequalities of patient access between Member States, between regions inside a Member State, between hospitals ÔÇó Slow placing on the market, can take several years for life saving drugs; no placing on the market for some medicines: ´éº No relation between the delay of placing on the market & patient availability, and the value of the drug for patients treatment ´éº Responsibilities of delays are on both sides: (a) Member States which either donÔÇÖt have the expertise on these rare diseases or just wish to save money (b) Pharmaceutical & Biotech companies which donÔÇÖt have the ressources to apply to over 27 different procedures in a short period of time or ar not interested by some too small national markets 9 Cluj-Napoca,26-27 June 2009
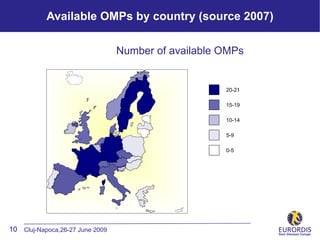
- 10. Available OMPs by country (source 2007) Number of available OMPs 20-21 15-19 10-14 5-9 0-5 10 Cluj-Napoca,26-27 June 2009
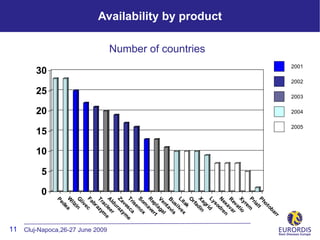
- 11. 2001 2002 2003 2004 2005 r ar ob ot Ph lt ia Pr m re Xy tio a ev r R va a ex n N dre Availability by product so Ly id gr Xa din Number of countries a rf O k ta x Li lve i us s B avi nt l Ve aga l t ep r R ave m x So no e is a T r sc e v e ym Za raz u ld r A lee e ac m 11 Cluj-Napoca,26-27 June 2009 Tr azy br Fa c e liv G in ilz W a de Pe 30 25 20 15 10 5 0
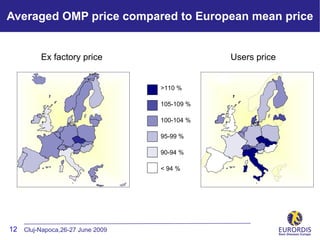
- 12. Averaged OMP price compared to European mean price Ex factory price Users price >110 % 105-109 % 100-104 % 95-99 % d 90-94 % < 94 % 12 Cluj-Napoca,26-27 June 2009
- 13. www.eurordis.org The European Common Scientific Assessment Of the Clinical Added Value of Orphan Drugs
- 14. The European Solution: From Agreed PrinciplesÔǪ ÔÇó New agreed principles (EU Pharma Forum): ´éº ┬½ Member States, stakeholders and the Commission should strenghten their efforts to ensure access to orphan medicines in all EU Member States ┬╗ ´éº EU Exchange of Knowledge on the Scientific Assessment of the Clinical Added Value of Orphan Medicines ´éº Specific Conditional Pricing & Reimbursement mechanisms ´éº Early dialogue on research & development ´éº Increased awareness on rare/orphan diseases 14 Cluj-Napoca,26-27 June 2009
- 15. The European Solution: ÔǪ to Action and new Good Practices ÔÇó New practices at EU Level (Commission Communication & Council Recommendation): ´éº European Collaboration for the Scientific Assessment of the Clinical Added Value of Orphan Medicines ´éº Creation of a Working Party to produce European Scientific Common Assessment Report on this Clinical Added Value ´éº Regular revision & update of clinical added value reports based on post-MA data from real life studies 15 Cluj-Napoca,26-27 June 2009
- 16. National Strategies or Plans on Rare Diseases: from EU agreed principles to concrete measures ÔÇó New practices at national level, including new EU Member states, in the next national strategies & plans on rare diseases 2009-2013 (and recommended to all European countries): ´éº Commitment to take active part in this new EU collaboration ´éº Commitment to use the European Common Assessment Report to speed up its national decision making process and to do its appraisal on Pricing & Reimbursement ´éº Promote Conditional Pricing & Reimbursement with regular revisions, based on revised & updated Reports ´éº National payment, even when prescribed and distributed in hospitals 16 Cluj-Napoca,26-27 June 2009
- 17. Why Romania and Balkan countries should be willing to implement these agreed principles and new practices? ÔÇó Because Romania and Balkan countries have taken part to the development of these new principles and have agreed to them (EU Pharma Forum and Council of Health Ministers of the European Union) ÔÇó Because Romania and Balkan countries would: ´éº Benefit from quicker, better quality and more consistent assessment thanks to their collaboration with other Member States: scientific and medical data on which clinical added value is assessed are the same and are valid across Europe ´éº Coordinate their post marketing requirements with other Member States, asking for real life studies or registries for instance, with higher chances of obtaining more rapidly more reliable data ´éº Be able to do have better value for its money when paying for orphan medicines thanks to (a) better medical practices & prescriptions (b) taking advantage of conditional pricing & reimbursement (c ) better targeted patients responsive to the treatment within the therapeutic indication 17 Cluj-Napoca,26-27 June 2009
- 18. www.eurordis.org Yann Le Cam Fabrizia Bignami François Houÿez Chief Executive Therapeutic Development Health Policy ylecam@eurordis.org fbignami@eurordis.org fhouyez@eurordis.org Flaminia Macchia Anja Helm Paloma Tejada European Public Affairs Relations with Patient Groups Communications fmacchia@eurordis.org ahelm@eurordis.org ptejada@eurordis.org 18 Cluj-Napoca,26-27 June 2009