Concentration of ions in the solution
Download as doc, pdf0 likes649 views
The document describes the electrolysis of a 1.0 mol/dm3 sodium chloride solution using carbon electrodes. During electrolysis, sodium and chloride ions move to the cathode and anode respectively. At the cathode, hydrogen ions are discharged to produce hydrogen gas while at the anode, chloride ions are discharged to produce chlorine gas. The solution becomes alkaline after electrolysis due to the presence of sodium hydroxide.
1 of 1
Downloaded 28 times
Ad
Recommended
Type of electrodes
Type of electrodesFaridah Hamat
Ėý
This document describes the electrolysis of a copper(II) sulfate solution using copper electrodes. During electrolysis, copper ions and hydrogen ions move to the cathode where copper ions are discharged to form a brown copper deposit, while hydroxide and sulfate ions move to the anode where the copper electrode dissolves to release copper ions and electrons. The process results in the cathode gaining mass and thickness while the anode loses mass and becomes thinner, but the concentration and blue color of the copper ions in the solution remains unchanged.Electrolysis molten sodium chloride
Electrolysis molten sodium chlorideFaridah Hamat
Ėý
The document summarizes the process of electrolysis for two types of electrolytes: molten sodium chloride and aqueous electrolytes. For molten sodium chloride, sodium and chloride ions move to the cathode and anode, respectively, where they are discharged. Sodium forms at the cathode while chlorine gas forms at the anode. For aqueous electrolytes like dilute sulfuric acid, hydrogen, sulfate, and hydroxide ions are present and migrate to the electrodes where hydrogen gas forms at the cathode and oxygen gas forms at the anode.6.2 (b) molten compound2
6.2 (b) molten compound2Azieda Dot
Ėý
Here are the key steps to identify products of electrolysis in molten compounds:
1. Identify the ions present in the molten compound
2. Determine which ions are attracted to the cathode and anode based on being positive or negative
3. Write the products formed by the ions discharging at each electrode
4. Write balanced half reactions for the cathode and anode
Following these steps allows you to determine the products of electrolysis for any molten compound.Describe electrolysis of an aqueous solution
Describe electrolysis of an aqueous solutionkaiying
Ėý
Electrolysis of an aqueous solution involves applying a current to induce a chemical reaction. Cations move to the cathode and anions move to the anode. At the cathode, silver ions are reduced to silver metal and copper ions are reduced to copper metal. At the anode, bromide ions are oxidized to bromine gas and hydroxide ions are oxidized to oxygen gas and water. The overall reactions produce silver and bromine from silver bromide and copper and oxygen from copper sulfate.6.3 (b) half equations
6.3 (b) half equationsAzieda Dot
Ėý
1. The document discusses the steps to predict the products of electrolysis of aqueous solutions by identifying the ions present, which ions move to the cathode and anode, and which ions will discharge at each electrode.
2. Half reactions are written for the electrolysis of diluted and concentrated hydrochloric acid that show hydrogen ions discharging at the cathode and chlorine or oxygen discharging at the anode.
3. Observations are described for experiments electrolyzing diluted and concentrated hydrochloric acid that are consistent with the predicted half reactions.ELECTROCHEMSTRY POWER POINT
ELECTROCHEMSTRY POWER POINTwanafifah
Ėý
Electrolysis is the process of using electric current to cause non-spontaneous chemical changes. During electrolysis, ions are discharged at the electrodes. The key factors that determine which ions are discharged include the position of ions in the electrochemical series, concentration of ions, and type of electrode. Electrolysis has important industrial applications such as electroplating, metal purification, and metal extraction.Electrolytic processes - C3.3 Heba Saey
Electrolytic processes - C3.3 Heba SaeyHebaalrifai
Ėý
This document provides information about electrolytic processes and electrolysis. It discusses key points about electrolysis, including that ions must be free to move towards electrodes. It then summarizes different types of electrolysis including molten salts like NaCl, aqueous solutions like NaCl, and using copper electrodes. Electroplating is also discussed as coating a metal with another using electricity, with examples like silver plating brass.Electricity and chemistry class 10
Electricity and chemistry class 10Sodhathshanavas S Bsos
Ėý
This document discusses the electrolysis of brine solution (concentrated NaCl) to produce sodium hydroxide and chlorine gas. During electrolysis, sodium and hydrogen ions move to the cathode while chlorine and hydroxide ions move to the anode. At the cathode, only hydrogen ions are discharged to form hydrogen gas. At the anode, chlorine ions are discharged to form chlorine gas, leaving behind a solution of sodium hydroxide. A diaphragm cell is used to separately collect the chlorine gas and sodium hydroxide solution produced. Sodium hydroxide has uses including in soap production and paper making. Chlorine gas has uses as a bleaching agent and to produce hydrochlorElectrolysis
Electrolysis Rossita Radzak
Ėý
The three main factors that affect the electrolysis of an aqueous solution are:
1) The position of ions in the electrochemical series, which determines which ions will be reduced or oxidized at the electrodes.
2) The concentration of ions or electrolytes in the solution. Higher concentrations result in greater rates of reaction.
3) The type of electrodes used, as certain electrodes may dissolve and alter the composition of the solution during electrolysis.Electrolysis
Electrolysis arahmohamed
Ėý
This document discusses electrolysis and electrochemical cells. It explains that during electrolysis, ions lower in the electrochemical series are more easily discharged. It also discusses how the concentration of ions and the nature of electrodes used can affect what products are formed. Specifically, it provides examples of electrolysis of copper sulfate solution and sodium chloride solution, and discusses how inert vs reactive electrodes and concentrated vs dilute solutions impact the results. It also summarizes how electrolysis can be used for electroplating, purifying copper, and in simple electrochemical cells like the Daniell cell.Electrolysis part 3 aqueous solution
Electrolysis part 3 aqueous solutionkaiying
Ėý
The document summarizes electrolysis of aqueous solutions. It defines electrolysis as the process of using an electric current to cause a non-spontaneous chemical reaction. It describes that during electrolysis, cations move to the cathode and are reduced, while anions move to the anode and are oxidized. Factors like the electrochemical series, ion concentration, and electrode type determine which ions are discharged. Products are identified based on half-reactions and observations like gas evolution and metal deposition.6.3 (a) electrolysis of an aqueous solution
6.3 (a) electrolysis of an aqueous solutionAzieda Dot
Ėý
The document discusses the electrolysis of aqueous solutions. It explains that during electrolysis, only one ion is selectively discharged at each electrode based on its position in the electrochemical series, the nature of the electrode, and the concentration of ions. The ion discharged at the anode depends on which is easier to oxidize, while the ion discharged at the cathode depends on which is easier to reduce. Different products are formed depending on these factors and the specific electrolyte used.Electrolysis of brine
Electrolysis of brineLaura Ortega
Ėý
The document provides detailed information on various chemicals derived from salt, including their compositions and applications. It explains the extraction processes of rock salt and brine, as well as the electrolysis procedure that produces sodium hydroxide, hydrogen, and chlorine. Additionally, it outlines the use of these products in food preservation, chemical manufacturing, and other industries.Electrolysis revision
Electrolysis revisionsue forshaw
Ėý
This document provides information about electrolysis. It discusses:
1. How electrolysis can be used to split up ionic compounds by melting them or dissolving them in water. Positive ions move to the negative cathode and negative ions move to the positive anode.
2. The rules for what products are formed at the electrodes during electrolysis of molten ionic compounds and ionic solutions. Hydrogen, metals, halogens, oxygen may be produced depending on the ions present.
3. Industrial applications of electrolysis including producing chlorine and sodium hydroxide from salt water, and purifying copper.Electrolysis part 2: molten compound
Electrolysis part 2: molten compoundkaiying
Ėý
1) Electrolysis of molten compounds involves passing an electric current through a molten compound, causing its ions to migrate to the electrodes.
2) During electrolysis of molten lead(II) bromide (PbBr2), the Pb2+ ions migrate to the cathode, where they gain electrons and deposit as metallic lead. Meanwhile, the Br- ions migrate to the anode, where they lose electrons and form a bromine gas product.
3) The overall reaction is the decomposition of PbBr2 into lead metal and bromine gas.Electrolysis molten substances
Electrolysis molten substancesPhang Chia Chen
Ėý
Electrolytes are substances that can conduct electricity in the molten or liquid state and undergo chemical changes. Electrolysis is a process where electrolytes are broken down into their constituent elements by passing electricity through them. During electrolysis, ions migrate to the oppositely charged electrodes. At the anode, ions lose electrons and form gases or dissolve. At the cathode, ions gain electrons and form solid elements. Examples of electrolysis of molten lead(II) bromide and lead(II) oxide are described through their half reactions at the anode and cathode and overall reactions.Powerpoint micro teaching
Powerpoint micro teachingNurul Isa
Ėý
The document discusses electrolysis of aqueous solutions. It explains that aqueous solutions contain anions, cations, and ions from the partial dissociation of water (H+ and OH-). Electrolysis of copper(II) sulfate solution results in copper metal depositing at the cathode and oxygen gas releasing at the anode. Several factors affect the products of electrolysis, including position of ions in the electrochemical series and concentration of electrolytes. The document concludes with an example of the half reactions during electrolysis of copper(II) sulfate solution.Electricity & Chemistry
Electricity & Chemistryguesta52c13
Ėý
The document discusses electrolysis and the principles behind it. It explains that electrolysis involves passing electricity through an electrolyte, which causes chemical decomposition. Ions migrate to the electrodes and are discharged. Metals are formed at the cathode by reduction, while non-metals or oxygen form at the anode by oxidation. It provides examples of electrolysis such as molten salts like NaCl and aqueous solutions like copper sulfate. Factors affecting ion discharge are also discussed.ELECTRICITY AND CHEMISTRY
ELECTRICITY AND CHEMISTRYINSTITUTO TECNOLÃGICO DE SONORA
Ėý
1. Electrolysis is the process of using electricity to cause non-spontaneous chemical changes.
2. During electrolysis, ions migrate towards the oppositely charged electrode - cations move towards the cathode and anions move towards the anode.
3. At the cathode, cations gain electrons and are reduced. At the anode, anions lose electrons and are oxidized.
4. The products of electrolysis depend on the electrolyte. Molten salts yield elements, while aqueous solutions yield hydrogen or oxygen along with other possible products.Std XI-Chapter-5-Redox-Reactions-Applications
Std XI-Chapter-5-Redox-Reactions-ApplicationsGurudatta Wagh
Ėý
The document discusses applications of redox reactions, including combustion of methane, bleaching with oxidizing agents, electron transfer in batteries, and metallurgy processes for zinc extraction. It also highlights the oxidation and reduction of various elements during these reactions. Additionally, it mentions the significance of respiration, where glucose oxidizes to release energy.C5 syllabus statements
C5 syllabus statementscartlidge
Ėý
This document outlines the key topics and concepts covered in a syllabus for a C5 Electricity & Chemistry course. It includes 9 points that describe electrolysis processes, products, and principles. Specifically, it covers how electrolysis breaks ionic compounds into simpler substances through the chemical effects of electricity. It also describes the use of electrodes, electrolytes, anodes and cathodes and how they relate to the reactions and products in different electrolysis examples, including molten salts, aqueous solutions, and metal refining. Finally, it mentions electroplating of metals and the industrial production of aluminum, chlorine, hydrogen and sodium hydroxide.Std XI-Ch-5-Redox-Reactions
Std XI-Ch-5-Redox-ReactionsGurudatta Wagh
Ėý
This document discusses oxidation numbers, which indicate the degree of oxidation or reduction of atoms in chemical compounds. Oxidation numbers are assigned according to rules such as atoms in their elemental form having an oxidation number of 0, monoatomic ions having the same charge as their oxidation number, and the sum of oxidation numbers in a neutral molecule being 0. Oxidation numbers can be fractional and are indicated in Roman numerals after the symbol of a metal in its compound. An example of a fractional oxidation number is given for sulfur atoms in the tetrathionate ion.Inorganic Chemistry : Electrochemistry
Inorganic Chemistry : ElectrochemistryThivyaapriya Sambamoorthy
Ėý
Dokumen ini membahas pengenalan dan konsep dasar elektrokimia, termasuk nomor oksidasi, reaksi redoks, dan cara menyeimbangkan reaksi tersebut. Nomor oksidasi digunakan untuk menentukan proses oksidasi dan reduksi suatu zat, dengan berbagai contoh reaksi kimia dan perubahan nomor oksidasi yang disertakan. Bagian akhir membahas metode lain untuk menyeimbangkan reaksi redoks dan aplikasi dalam kondisi basa.Redox reactions
Redox reactionsarahmohamed
Ėý
This document discusses redox reactions and oxidation states. It defines oxidizing and reducing agents as chemical species that cause the other reactant to be oxidized or reduced, respectively. Redox reactions involve the oxidation of one substance and the reduction of another through electron transfer. Tests are described to identify reducing and oxidizing agents based on their ability to reduce dichromate, manganate, or oxidize iodide ions. The document also explains how to determine the oxidation state of atoms in compounds and polyatomic ions using the fact that the sum of oxidation states equals the overall charge.Marking scheme-chemistry-perfect-score-module-form-4-set-3
Marking scheme-chemistry-perfect-score-module-form-4-set-3Mudzaffar Shah
Ėý
The document provides information on three electrolysis experiments involving different electrolytes and products observed at the anode and cathode. Experiment 1 uses sodium chloride solution, with chlorine gas produced at the anode and hydrogen gas at the cathode. Experiment 2 uses hydrochloric acid, producing oxygen gas at the anode and hydrogen gas at the cathode. Experiment 3 uses copper sulfate solution, with no ions being discharged and copper metal being deposited at the cathode through the oxidation of copper electrodes.Chemistry perfect-score-module-form-4-set-3
Chemistry perfect-score-module-form-4-set-3Mudzaffar Shah
Ėý
1) Electrolysis involves passing an electric current through an electrolyte to cause non-spontaneous redox reactions to occur at the electrodes.
2) Products of electrolysis depend on the nature of the electrolyte and electrodes. Selective discharge of ions occurs based on their position in the electrochemical series.
3) Voltaic cells involve spontaneous redox reactions that generate electricity, with electrons flowing from the negative to the positive terminal.Ionic bonding
Ionic bondingMiles Cawley
Ėý
Ionic bonds form when a metal transfers an electron to a nonmetal, giving each atom an octet of electrons. For example, sodium loses an electron to form Na+ while chlorine gains that electron to form Cl-. The resulting ions are held together by electrostatic attraction to form an ionic compound, sodium chloride (NaCl). NaCl crystallizes into a repeating pattern where Na+ and Cl- ions alternate in a crystal lattice. Ionic compounds conduct electricity when molten or dissolved due to the movement of ions.Redox presentation 11 july 2011
Redox presentation 11 july 2011MRSMPC
Ėý
This document defines redox reactions as processes where electrons are either gained (reduction) or lost (oxidation). It provides examples of calculating oxidation states and naming ionic compounds. It then discusses a redox reaction between iron(II) chloride and chlorine, writing balanced equations and identifying oxidizing/reducing agents. Finally, it covers a redox reaction between iodide and dichromate ions, including half and overall equations.Round robin english
Round robin englishFaridah Hamat
Ėý
This document contains information about chemistry concepts taught using the "Round Robin" method. It includes 8 sections called PINTAR that cover topics like solubility rules for salts, reactions to form salts, tests to identify cations, and the behavior of salts when heated. The purpose is for students to memorize the content through repeated oral recitation of the sections in a round-robin style until all students have memorized the full content.Bengkel elektrolisis 2
Bengkel elektrolisis 2Faridah Hamat
Ėý
The document provides information about electrolysis experiments using different electrolyte solutions and electrodes. In the first experiment, a sodium sulfate solution is electrolyzed using carbon electrodes. Ions move to the electrodes and gases are produced. Hydrogen gas is collected at one electrode. In the second experiment, dilute sodium chloride solution is electrolyzed and chlorine gas is collected at one electrode.More Related Content
What's hot (20)
Electrolysis
Electrolysis Rossita Radzak
Ėý
The three main factors that affect the electrolysis of an aqueous solution are:
1) The position of ions in the electrochemical series, which determines which ions will be reduced or oxidized at the electrodes.
2) The concentration of ions or electrolytes in the solution. Higher concentrations result in greater rates of reaction.
3) The type of electrodes used, as certain electrodes may dissolve and alter the composition of the solution during electrolysis.Electrolysis
Electrolysis arahmohamed
Ėý
This document discusses electrolysis and electrochemical cells. It explains that during electrolysis, ions lower in the electrochemical series are more easily discharged. It also discusses how the concentration of ions and the nature of electrodes used can affect what products are formed. Specifically, it provides examples of electrolysis of copper sulfate solution and sodium chloride solution, and discusses how inert vs reactive electrodes and concentrated vs dilute solutions impact the results. It also summarizes how electrolysis can be used for electroplating, purifying copper, and in simple electrochemical cells like the Daniell cell.Electrolysis part 3 aqueous solution
Electrolysis part 3 aqueous solutionkaiying
Ėý
The document summarizes electrolysis of aqueous solutions. It defines electrolysis as the process of using an electric current to cause a non-spontaneous chemical reaction. It describes that during electrolysis, cations move to the cathode and are reduced, while anions move to the anode and are oxidized. Factors like the electrochemical series, ion concentration, and electrode type determine which ions are discharged. Products are identified based on half-reactions and observations like gas evolution and metal deposition.6.3 (a) electrolysis of an aqueous solution
6.3 (a) electrolysis of an aqueous solutionAzieda Dot
Ėý
The document discusses the electrolysis of aqueous solutions. It explains that during electrolysis, only one ion is selectively discharged at each electrode based on its position in the electrochemical series, the nature of the electrode, and the concentration of ions. The ion discharged at the anode depends on which is easier to oxidize, while the ion discharged at the cathode depends on which is easier to reduce. Different products are formed depending on these factors and the specific electrolyte used.Electrolysis of brine
Electrolysis of brineLaura Ortega
Ėý
The document provides detailed information on various chemicals derived from salt, including their compositions and applications. It explains the extraction processes of rock salt and brine, as well as the electrolysis procedure that produces sodium hydroxide, hydrogen, and chlorine. Additionally, it outlines the use of these products in food preservation, chemical manufacturing, and other industries.Electrolysis revision
Electrolysis revisionsue forshaw
Ėý
This document provides information about electrolysis. It discusses:
1. How electrolysis can be used to split up ionic compounds by melting them or dissolving them in water. Positive ions move to the negative cathode and negative ions move to the positive anode.
2. The rules for what products are formed at the electrodes during electrolysis of molten ionic compounds and ionic solutions. Hydrogen, metals, halogens, oxygen may be produced depending on the ions present.
3. Industrial applications of electrolysis including producing chlorine and sodium hydroxide from salt water, and purifying copper.Electrolysis part 2: molten compound
Electrolysis part 2: molten compoundkaiying
Ėý
1) Electrolysis of molten compounds involves passing an electric current through a molten compound, causing its ions to migrate to the electrodes.
2) During electrolysis of molten lead(II) bromide (PbBr2), the Pb2+ ions migrate to the cathode, where they gain electrons and deposit as metallic lead. Meanwhile, the Br- ions migrate to the anode, where they lose electrons and form a bromine gas product.
3) The overall reaction is the decomposition of PbBr2 into lead metal and bromine gas.Electrolysis molten substances
Electrolysis molten substancesPhang Chia Chen
Ėý
Electrolytes are substances that can conduct electricity in the molten or liquid state and undergo chemical changes. Electrolysis is a process where electrolytes are broken down into their constituent elements by passing electricity through them. During electrolysis, ions migrate to the oppositely charged electrodes. At the anode, ions lose electrons and form gases or dissolve. At the cathode, ions gain electrons and form solid elements. Examples of electrolysis of molten lead(II) bromide and lead(II) oxide are described through their half reactions at the anode and cathode and overall reactions.Powerpoint micro teaching
Powerpoint micro teachingNurul Isa
Ėý
The document discusses electrolysis of aqueous solutions. It explains that aqueous solutions contain anions, cations, and ions from the partial dissociation of water (H+ and OH-). Electrolysis of copper(II) sulfate solution results in copper metal depositing at the cathode and oxygen gas releasing at the anode. Several factors affect the products of electrolysis, including position of ions in the electrochemical series and concentration of electrolytes. The document concludes with an example of the half reactions during electrolysis of copper(II) sulfate solution.Electricity & Chemistry
Electricity & Chemistryguesta52c13
Ėý
The document discusses electrolysis and the principles behind it. It explains that electrolysis involves passing electricity through an electrolyte, which causes chemical decomposition. Ions migrate to the electrodes and are discharged. Metals are formed at the cathode by reduction, while non-metals or oxygen form at the anode by oxidation. It provides examples of electrolysis such as molten salts like NaCl and aqueous solutions like copper sulfate. Factors affecting ion discharge are also discussed.ELECTRICITY AND CHEMISTRY
ELECTRICITY AND CHEMISTRYINSTITUTO TECNOLÃGICO DE SONORA
Ėý
1. Electrolysis is the process of using electricity to cause non-spontaneous chemical changes.
2. During electrolysis, ions migrate towards the oppositely charged electrode - cations move towards the cathode and anions move towards the anode.
3. At the cathode, cations gain electrons and are reduced. At the anode, anions lose electrons and are oxidized.
4. The products of electrolysis depend on the electrolyte. Molten salts yield elements, while aqueous solutions yield hydrogen or oxygen along with other possible products.Std XI-Chapter-5-Redox-Reactions-Applications
Std XI-Chapter-5-Redox-Reactions-ApplicationsGurudatta Wagh
Ėý
The document discusses applications of redox reactions, including combustion of methane, bleaching with oxidizing agents, electron transfer in batteries, and metallurgy processes for zinc extraction. It also highlights the oxidation and reduction of various elements during these reactions. Additionally, it mentions the significance of respiration, where glucose oxidizes to release energy.C5 syllabus statements
C5 syllabus statementscartlidge
Ėý
This document outlines the key topics and concepts covered in a syllabus for a C5 Electricity & Chemistry course. It includes 9 points that describe electrolysis processes, products, and principles. Specifically, it covers how electrolysis breaks ionic compounds into simpler substances through the chemical effects of electricity. It also describes the use of electrodes, electrolytes, anodes and cathodes and how they relate to the reactions and products in different electrolysis examples, including molten salts, aqueous solutions, and metal refining. Finally, it mentions electroplating of metals and the industrial production of aluminum, chlorine, hydrogen and sodium hydroxide.Std XI-Ch-5-Redox-Reactions
Std XI-Ch-5-Redox-ReactionsGurudatta Wagh
Ėý
This document discusses oxidation numbers, which indicate the degree of oxidation or reduction of atoms in chemical compounds. Oxidation numbers are assigned according to rules such as atoms in their elemental form having an oxidation number of 0, monoatomic ions having the same charge as their oxidation number, and the sum of oxidation numbers in a neutral molecule being 0. Oxidation numbers can be fractional and are indicated in Roman numerals after the symbol of a metal in its compound. An example of a fractional oxidation number is given for sulfur atoms in the tetrathionate ion.Inorganic Chemistry : Electrochemistry
Inorganic Chemistry : ElectrochemistryThivyaapriya Sambamoorthy
Ėý
Dokumen ini membahas pengenalan dan konsep dasar elektrokimia, termasuk nomor oksidasi, reaksi redoks, dan cara menyeimbangkan reaksi tersebut. Nomor oksidasi digunakan untuk menentukan proses oksidasi dan reduksi suatu zat, dengan berbagai contoh reaksi kimia dan perubahan nomor oksidasi yang disertakan. Bagian akhir membahas metode lain untuk menyeimbangkan reaksi redoks dan aplikasi dalam kondisi basa.Redox reactions
Redox reactionsarahmohamed
Ėý
This document discusses redox reactions and oxidation states. It defines oxidizing and reducing agents as chemical species that cause the other reactant to be oxidized or reduced, respectively. Redox reactions involve the oxidation of one substance and the reduction of another through electron transfer. Tests are described to identify reducing and oxidizing agents based on their ability to reduce dichromate, manganate, or oxidize iodide ions. The document also explains how to determine the oxidation state of atoms in compounds and polyatomic ions using the fact that the sum of oxidation states equals the overall charge.Marking scheme-chemistry-perfect-score-module-form-4-set-3
Marking scheme-chemistry-perfect-score-module-form-4-set-3Mudzaffar Shah
Ėý
The document provides information on three electrolysis experiments involving different electrolytes and products observed at the anode and cathode. Experiment 1 uses sodium chloride solution, with chlorine gas produced at the anode and hydrogen gas at the cathode. Experiment 2 uses hydrochloric acid, producing oxygen gas at the anode and hydrogen gas at the cathode. Experiment 3 uses copper sulfate solution, with no ions being discharged and copper metal being deposited at the cathode through the oxidation of copper electrodes.Chemistry perfect-score-module-form-4-set-3
Chemistry perfect-score-module-form-4-set-3Mudzaffar Shah
Ėý
1) Electrolysis involves passing an electric current through an electrolyte to cause non-spontaneous redox reactions to occur at the electrodes.
2) Products of electrolysis depend on the nature of the electrolyte and electrodes. Selective discharge of ions occurs based on their position in the electrochemical series.
3) Voltaic cells involve spontaneous redox reactions that generate electricity, with electrons flowing from the negative to the positive terminal.Ionic bonding
Ionic bondingMiles Cawley
Ėý
Ionic bonds form when a metal transfers an electron to a nonmetal, giving each atom an octet of electrons. For example, sodium loses an electron to form Na+ while chlorine gains that electron to form Cl-. The resulting ions are held together by electrostatic attraction to form an ionic compound, sodium chloride (NaCl). NaCl crystallizes into a repeating pattern where Na+ and Cl- ions alternate in a crystal lattice. Ionic compounds conduct electricity when molten or dissolved due to the movement of ions.Redox presentation 11 july 2011
Redox presentation 11 july 2011MRSMPC
Ėý
This document defines redox reactions as processes where electrons are either gained (reduction) or lost (oxidation). It provides examples of calculating oxidation states and naming ionic compounds. It then discusses a redox reaction between iron(II) chloride and chlorine, writing balanced equations and identifying oxidizing/reducing agents. Finally, it covers a redox reaction between iodide and dichromate ions, including half and overall equations.Viewers also liked (10)
Round robin english
Round robin englishFaridah Hamat
Ėý
This document contains information about chemistry concepts taught using the "Round Robin" method. It includes 8 sections called PINTAR that cover topics like solubility rules for salts, reactions to form salts, tests to identify cations, and the behavior of salts when heated. The purpose is for students to memorize the content through repeated oral recitation of the sections in a round-robin style until all students have memorized the full content.Bengkel elektrolisis 2
Bengkel elektrolisis 2Faridah Hamat
Ėý
The document provides information about electrolysis experiments using different electrolyte solutions and electrodes. In the first experiment, a sodium sulfate solution is electrolyzed using carbon electrodes. Ions move to the electrodes and gases are produced. Hydrogen gas is collected at one electrode. In the second experiment, dilute sodium chloride solution is electrolyzed and chlorine gas is collected at one electrode.Electrolyte
ElectrolyteFaridah Hamat
Ėý
Electrolytes are substances that can conduct electricity when molten or dissolved in water, as they dissociate into ions that are free to move. Non-electrolytes do not contain ions and cannot conduct electricity either when molten or dissolved. While solid ionic compounds cannot conduct due to their tightly bound ions, they can conduct in molten or aqueous states as their ions gain freedom of movement.Electrolysis of molten compounds
Electrolysis of molten compoundsFaridah Hamat
Ėý
Electrolysis is a process where an electrolyte is broken down into its constituent elements when electricity passes through. An electrolytic cell converts electrical energy to chemical energy using a battery, electrolyte, and two electrodes. During electrolysis, anions move toward the anode and cations move toward the cathode, where they are discharged by gaining or losing electrons.Acid And Base
Acid And BaseFaridah Hamat
Ėý
An acid is a chemical substance that produces hydrogen ions (H+) in water, while a base reacts with an acid to produce salt and water. Strong acids fully ionize in water to produce a high concentration of H+ ions, while weak acids only partially ionize, producing a low H+ concentration. Strong bases fully ionize to produce a high concentration of hydroxide (OH-) ions, unlike weak bases. Acids have sour tastes, turn litmus paper red, and are electrolytes in water. They react with metals, bases, carbonates, and alkalis to produce salts, hydrogen gas, water, and carbon dioxide. Bases have bitter tastes, turn litmus paper blue, and are electrolytesBuffer Systems and Titration
Buffer Systems and Titrationaqion
Ėý
The document discusses the mathematical background of buffer systems and alkalimetric titration, focusing on diprotic acids such as carbonic acid in equilibrium with strong bases. It covers the relationships between pH, concentrations of species, and the effects of ionic strength on buffer intensity, including formulas and derivations. Additionally, it addresses the optimal buffer ranges and the influence of water's self-ionization on the system's behavior.Composite Carbonic Acid and Carbonate Kinetics
Composite Carbonic Acid and Carbonate Kineticsaqion
Ėý
There are two types of carbonic acid: true carbonic acid (H2CO3) and composite carbonic acid (H2CO3*). True carbonic acid involves the direct reaction of CO2 and H2O, while composite carbonic acid treats CO2(aq) and H2CO3 as a single entity. Each acid has its own equilibrium constant (Ktrue and K1). K1 is around 500 times smaller than Ktrue due to the inclusion of dissolved CO2 in the composite definition. The reaction kinetics between the three species (CO2(aq), H2CO3, HCO3-) were also described, noting that the direct reaction between CO2 and H2O is much slower thanChapter 9 manufacture substances
Chapter 9 manufacture substancesFaridah Hamat
Ėý
This document discusses several important industrial products and manufactured substances. It provides details on the production and uses of sulphuric acid, ammonia, alloys, synthetic polymers, glass, ceramics, and composite materials. Sulphuric acid is manufactured through the contact process and is used to make fertilizers and batteries. Ammonia is produced via the Haber process and converted into fertilizers. Alloys are metal mixtures that are stronger than pure metals. Glass and ceramics are hard, brittle materials made from sand and clay, respectively. Composite materials combine substances like metals and polymers to produce materials with improved properties.Chapter 2 structure of atom
Chapter 2 structure of atomFaridah Hamat
Ėý
1. The document provides lesson notes on the structure of the atom, including the particulate nature of matter, states of matter, atomic structure, isotopes, and the electronic structure of atoms.
2. It explains key concepts such as the kinetic particle theory, processes of changing state, diffusion, the historical development of atomic models, subatomic particles, and electron configuration.
3. Examples are given to illustrate isotopes and the arrangement of electrons in shells, with the first shell holding up to 2 electrons and the second up to 8.Chapter 4 perodic table
Chapter 4 perodic tableFaridah Hamat
Ėý
The document summarizes key aspects of the periodic table, including:
1) It describes the historical development of the periodic table by scientists like Lavoisier, Dobereiner, Newlands, Meyer, and Mendeleev.
2) It explains the modern arrangement of elements in the periodic table based on proton number and discusses the properties of elements in the same group and period.
3) It provides examples of properties and reactions of representative elements from groups 1, 17, 18 and period 3 of the periodic table. Transition elements and semimetals are also discussed.Ad
Similar to Concentration of ions in the solution (20)
ELECTROCHEMITRY
ELECTROCHEMITRYzlem
Ėý
Electrolysis is the process of using electric current to cause non-spontaneous chemical changes. During electrolysis, ions are discharged at the electrodes. The key factors that determine which ions are discharged include the position of ions in the electrochemical series, concentration of ions, and type of electrode. Electrolysis has various industrial applications including electroplating, metal purification, and metal extraction.C6d Chemistry Of Sodium Chloride
C6d Chemistry Of Sodium ChlorideM F Ebden
Ėý
Sodium chloride is used to make chlorine, sodium hydroxide, and hydrogen through industrial processes. It is an important raw material extracted from underground salt deposits through solution mining. Electrolysis of a sodium chloride solution produces chlorine gas at the anode, hydrogen gas at the cathode, and sodium hydroxide in the solution.Electrochemistry-Dr. Surendran Parambadath
Electrochemistry-Dr. Surendran ParambadathSurendran Parambadath
Ėý
The document discusses different types of electrochemical cells including primary cells that produce electricity from non-reversible chemical reactions and secondary cells that can be recharged by passing electricity in the opposite direction of the spontaneous reaction. Examples of primary cells discussed include Daniel, mercury, dry, and alkaline cells, while examples of secondary cells include lead-acid, nickel-cadmium, nickel-metal hydride, and lithium-ion batteries. The working and reactions of common cells like lead-acid, alkaline, and dry cells are also explained.C3f Electrolysis
C3f ElectrolysisM F Ebden
Ėý
This document discusses several examples of electrolysis processes:
1) Electrolysis of sodium chloride solution produces chlorine gas at the anode and sodium hydroxide solution at the cathode. Chlorine is used to make chemicals and disinfect water.
2) Electrolysis of sulfuric acid produces hydrogen gas at the cathode and oxygen gas at the anode.
3) Aluminum is extracted from its ore, bauxite, through electrolysis. Molten aluminum oxide is electrolyzed to produce aluminum metal at the cathode and oxygen gas at the anode.C5b Electrolysis
C5b ElectrolysisM F Ebden
Ėý
The document discusses electrolysis of molten and aqueous compounds. During electrolysis, ions are attracted to the electrodes and gain or lose electrons to form elements or different compounds. For molten compounds to electrolyze, they must decompose into ions. The ions then move towards the positively charged anode, where they lose electrons, and towards the negatively charged cathode, where they gain electrons. For aqueous solutions, the ions from water may plate out at the electrodes instead of ions from the dissolved compound. The amount of product formed depends on the charge transferred, which is calculated using current and time.Electrolysis.pptx
Electrolysis.pptxSabrinaDeBellotte1
Ėý
This document discusses factors that determine which ions are discharged at the electrodes during electrolysis. For molten compounds containing one cation and one anion, the cation will be reduced at the cathode and the anion will be oxidized at the anode. For compounds in solution, the position of ions in the electrochemical series and concentration determine which ions are discharged. Ions higher in the anion series and lower in the metal series are preferentially discharged at the anode and cathode respectively. In dilute solutions, OH- and H+ ions are discharged but in concentrated solutions the ions from the compound may be discharged instead.Sodium group
Sodium groupgiovanniveitch
Ėý
The document discusses the Downs Process for extracting sodium through the electrolysis of molten sodium chloride, where sodium ions are reduced to sodium atoms at the cathode and chlorine gas is produced at the anode. It also describes how sodium hydroxide is produced through the electrolysis of sodium chloride solutions, and lists some common uses of sodium and sodium hydroxide such as in soap production, as a drain cleaner, and for lighting.Electrolysis
ElectrolysisAmanSaini51077
Ėý
Electrolysis is a process that uses electricity to separate substances. When an ionic substance is melted or dissolved in water, ions are free to move and an electric current can break them down into elements. During electrolysis, positive ions move to the negative cathode where they gain electrons and negative ions move to the positive anode where they lose electrons. Electrolysis of brine produces chlorine gas, hydrogen gas, and sodium hydroxide solution which have important industrial uses.Water
Water Nidhi D
Ėý
The document discusses the molecular structure of water. It explains that a water molecule is formed by two hydrogen atoms and one oxygen atom bonded together at an angle of 104.5 degrees. The polarity of the water molecule arises from oxygen's higher electronegativity which gives it a partial negative charge and the hydrogen atoms a partial positive charge. This polar nature is an important feature of water and causes it to have unique properties.ELECTROLYSIS PPT.FDGFYD UDRSECVXF KYS GDTSUTS
ELECTROLYSIS PPT.FDGFYD UDRSECVXF KYS GDTSUTSMalikKalim2
Ėý
The document explains the process of electrolysis, detailing how ionic compounds can conduct electricity when dissolved in a solution due to the movement of charged ions. It outlines the roles of anodes and cathodes, and the chemical reactions that occur at each electrode during electrolysis, including oxidation and reduction processes. Additionally, it provides examples of various ionic compounds and the products formed at the electrodes during the electrolysis process.ELECTROLYSIS.pdf
ELECTROLYSIS.pdfBagalanaSteven
Ėý
1. Electricity can affect substances in different ways depending on whether they are conductors, insulators, electrolytes or non-electrolytes. Conductors allow electric current to pass through due to mobile electrons, while insulators do not due to electrons locked in bonds.
2. Electrolysis is the decomposition of an electrolyte solution or melt by passing an electric current through it. At the anode, oxidation occurs as ions lose electrons. At the cathode, reduction occurs as ions gain electrons. Common electrolytes decomposed include NaCl, CuSO4 and acidified water.
3. During the electrolysis of molten lead(II) bromide, lead metal deposits at the cathode while bromineAnswers
AnswersCikgu Shaiful Kisas
Ėý
The document discusses electrolysis experiments using two electrodes, P and Q. At electrode P/cathode, sodium and hydrogen ions are attracted due to their position in the electrochemical series. At electrode Q/anode, chloride and hydroxide ions are attracted due to their higher concentration, and chloride ions are selectively discharged. The explanation involves experiments comparing the reactivity of metals like silver, L, and M based on their positions in the electrochemical series.2012 topic 4.1 bonding - ionic
2012 topic 4.1 bonding - ionicDavid Young
Ėý
The document discusses ionic bonding. Ionic bonds form between elements when one atom loses electrons to become a positively charged cation and another atom gains those electrons to become a negatively charged anion. This transfer of electrons allows both atoms to achieve a stable noble gas electron configuration. The resulting ions are held together by electrostatic attraction in a crystal lattice structure. Ionic compounds have high melting points, are brittle, and do not conduct electricity as solids since the ions are tightly bound. They dissolve in water, allowing the ions to separate and move, making the solutions electrically conductive.Ionic theory and electrolysis
Ionic theory and electrolysisMussaOmary3
Ėý
1) Electrolysis is the decomposition of electrolytes by the passage of an electric current through it. During electrolysis, ions move to the oppositely charged electrodes - cations to the cathode and anions to the anode.
2) At the anode, anions lose electrons in an oxidation reaction. At the cathode, cations gain electrons in a reduction reaction.
3) Which ion is discharged depends on factors like their position in the electrochemical series, concentration, and the electrode material. Ions higher in concentration or lower in the series will preferentially discharge.Acids and bases dr.surendran prambadath
Acids and bases dr.surendran prambadathSurendran Parambadath
Ėý
The document discusses acids and bases according to different theories including Arrhenius, Bronsted-Lowry, and Lewis concepts. It defines acids and bases, describes their properties, and explains neutralization reactions. Examples are provided of strong vs weak acids and bases as well as monoprotic, diprotic, and triprotic acids and bases based on their equivalent weights.Electrolysis of water
Electrolysis of watercgraham6
Ėý
The document describes an experiment on the electrolysis of water, detailing the process of decomposing water into oxygen and hydrogen using an electric current and sodium carbonate as a catalyst. It outlines pre-lab questions, step-by-step procedural instructions, and subsequent testing of the gases produced. Additionally, it includes reflection questions aimed at understanding the volumes of gases produced and their relationship to the composition of water.Electrolysis chemist
Electrolysis chemistHilmi Qimie
Ėý
Electrolysis is the process of using electricity to cause a non-spontaneous chemical reaction. During electrolysis, ions migrate towards the electrodes and undergo oxidation or reduction reactions. At the cathode, positively charged ions are reduced and neutral atoms are formed. At the anode, negatively charged ions are oxidized and neutral atoms or molecules are formed. The products of electrolysis depend on factors like the ions present in the electrolyte, their concentration and position in the reactivity series, and the nature of the electrodes. Electrolysis has applications in purifying and extracting metals, and in electroplating.Synthesis of Sodium metal
Synthesis of Sodium metalabdullahahmed223
Ėý
1. The Down's cell is an electrolytic cell that operates at 800°C to produce sodium metal through the electrolysis of molten sodium chloride.
2. It consists of a graphite anode and iron cathode separated by an iron screen inside a lined steel chamber. Molten sodium chloride mixed with calcium chloride and barium chloride is used as the electrolyte.
3. During electrolysis, chloride ions are oxidized at the anode to produce chlorine gas while sodium ions are reduced at the cathode to produce molten sodium metal. Some calcium is also produced but separates from the sodium due to differences in density.presentation1-190429202206.pdf
presentation1-190429202206.pdfJam Saeed Ahmed
Ėý
1. The Down's cell is an electrolytic cell that operates at 800°C to produce sodium metal through the electrolysis of molten sodium chloride.
2. It consists of a graphite anode and iron cathode separated by an iron screen inside a lined steel chamber. Molten sodium chloride mixed with calcium chloride and barium chloride is used as the electrolyte.
3. During electrolysis, chloride ions are oxidized at the anode to produce chlorine gas while sodium ions are reduced at the cathode to produce molten sodium metal. Some calcium is also produced but separates from the sodium due to differences in density.Electrolysis
Electrolysislisa14hazel
Ėý
Electrolysis is the decomposition of a substance by an electric current, where electrolytes carry current as ions in solution. During electrolysis, ions move to the electrodes and undergo oxidation or reduction reactions. At the cathode, electrons are gained and reduction occurs. At the anode, electrons are lost and oxidation occurs. The amount of substance deposited or gas produced can be calculated using Faraday's law, relating current, time, and moles of electrons in the electrode reactions.Ad
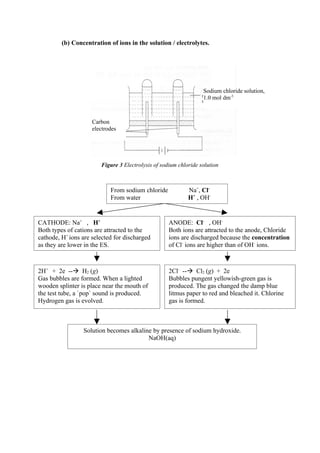
Concentration of ions in the solution
- 1. (b) Concentration of ions in the solution / electrolytes. Sodium chloride solution, 1.0 mol dm-3 Carbon electrodes Figure 3 Electrolysis of sodium chloride solution From sodium chloride Na+, Cl- From water H+ , OH- CATHODE: Na+ , H+ ANODE: Cl- , OH- Both types of cations are attracted to the Both ions are attracted to the anode, Chloride cathode, H+ ions are selected for discharged ions are discharged because the concentration as they are lower in the ES. of Cl- ions are higher than of OH- ions. 2H+ + 2e --ï H2 (g) 2Cl- --ï Cl2 (g) + 2e Gas bubbles are formed. When a lighted Bubbles pungent yellowish-green gas is wooden splinter is place near the mouth of produced. The gas changed the damp blue the test tube, a `pop` sound is produced. litmus paper to red and bleached it. Chlorine Hydrogen gas is evolved. gas is formed. Solution becomes alkaline by presence of sodium hydroxide. NaOH(aq)