Crystal field theory
Download as PPT, PDF57 likes61,538 views
Crystal Field Theory explains the colors of transition metal complexes based on ligand-metal interactions. The electrostatic interaction between ligands and metal d-orbitals splits the d-orbital energies. For an octahedral complex, the d-orbitals point directly at ligands have higher energy than those that bisect ligands. This splitting pattern determines if the complex is high or low spin, which then dictates its color and magnetic properties. The spectrochemical series orders ligands by their ability to cause crystal field splitting, correlating ligand type with complex color.
1 of 19
Downloaded 1,211 times

![High Spin Vs. Low Spin (d 1 to d 10 ) Electron Configuration for Octahedral complexes of metal ion having d 1 to d 10 configuration [M(H 2 O) 6 ] +n . Only the d 4 through d 7 cases have both high-spin and low spin configuration . Electron configurations for octahedral complexes of metal ions having from d 1 to d 10 configurations. Only the d 4 through d 7 cases have both high-spin and low-spin configurations.](https://image.slidesharecdn.com/crystalfieldtheory-111204095834-phpapp01/85/Crystal-field-theory-10-320.jpg)
![Color Absorption of Co 3+ Complexes The Colors of Some Complexes of the Co 3+ Ion The complex with fluoride ion, [CoF 6 ] 3+ , is high spin and has one absorption band. The other complexes are low spin and have two absorption bands. In all but one case, one of these absorptionsis in the visible region of the spectrum. The wavelengths refer to the center of that absorption band. Complex Ion Wavelength of Color of Light Color of Complex light absorbed Absorbed [CoF 6 ] 3+ 700 (nm) Red Green [Co(C 2 O 4 ) 3 ] 3+ 600, 420 Yellow, violet Dark green [Co(H 2 O) 6 ] 3+ 600, 400 Yellow, violet Blue-green [Co(NH 3 ) 6 ] 3+ 475, 340 Blue, violet Yellow-orange [Co(en) 3 ] 3+ 470, 340 Blue, ultraviolet Yellow-orange [Co(CN) 6 ] 3+ 310 Ultraviolet Pale Yellow](https://image.slidesharecdn.com/crystalfieldtheory-111204095834-phpapp01/85/Crystal-field-theory-11-320.jpg)
![Complex Influence on Color Compounds of Transition metal complexes solution. [Fe(H 2 O) 6 ] 3+ [Co(H 2 O) 6 ] 2+ [Ni(H 2 O) 6 ] 2+ [Cu(H 2 O) 6 ] 2+ [Zn(H 2 O) 6 ] 2+ 800 430 650 580 560 490 400](https://image.slidesharecdn.com/crystalfieldtheory-111204095834-phpapp01/85/Crystal-field-theory-16-320.jpg)
![Color Absorption of Co 3+ Complexes The Colors of Some Complexes of the Co 3+ Ion The complex with fluoride ion, [CoF 6 ] 3+ , is high spin and has one absorption band. The other complexes are low spin and have two absorption bands. In all but one case, one of these absorptionsis in the visible region of the spectrum. The wavelengths refer to the center of that absorption band.](https://image.slidesharecdn.com/crystalfieldtheory-111204095834-phpapp01/85/Crystal-field-theory-17-320.jpg)
Recommended
CFT



CFTbchandrakant
Ėý
The document summarizes key points about crystal field theory and its application to octahedral complexes. It discusses the historical development of metal complexes, assumptions of crystal field theory, and how it can be applied to explain splitting of d-orbitals in an octahedral complex. It also examines factors that affect crystal field stabilization energy, including the nature of the metal ion and ligands. Finally, it describes how crystal field theory can be used to understand the color and magnetic properties of complexes.Crystal Field Theory (CFT)



Crystal Field Theory (CFT)ÚاÚĐŲđØą Ų
ØŲ
ØŊ اØģŲŲ
ØđØ·Ø§ØąÛ
Ėý
Crystal field theory proposes that ligands behave as point charges that create an electric field around a central metal ion. This affects the energies of the metal's d-orbitals. In an octahedral complex, ligands along the x, y, and z axes interact more strongly with the dz2 and dx2-y2 orbitals, splitting them into the higher-energy eg set. The dxy, dyz, and dxz orbitals interact less with ligands between the axes, forming the lower-energy t2g set. This splitting of orbital energies, described by the crystal field splitting parameter Î0, helps explain differences in complexes' magnetic properties.Schrodinger Equation of Hydrogen Atom



Schrodinger Equation of Hydrogen AtomSaad Shaukat
Ėý
The document discusses the Schrodinger equation of the hydrogen atom. It shows how the Schrodinger equation can be separated into radial and angular variables using spherical coordinates. This results in three ordinary differential equations - one for the radial coordinate and two for the angular coordinates. The solutions of these equations involve quantum numbers such as the orbital angular momentum quantum number l and its magnetic quantum number ml.Transition Elements



Transition ElementsAssistant Professor in Chemistry
Ėý
This document discusses transition series elements and their properties. It describes how transition elements have electrons that enter the (n-1)d orbitals, giving them variable oxidation states up to +8. Their atomic radii decrease across periods but increase down groups. Transition metals can conduct heat and electricity well and can be alloyed to improve strength. Some have magnetic properties depending on unpaired electrons. Their colored complexes are due to electron transitions between d orbitals. Common applications include stainless steel, bronze, and uses of copper and nickel in coins, batteries, and turbines.Crystal field stabilization energy



Crystal field stabilization energyamna khalid
Ėý
This document discusses crystal field stabilization energy (CFSE), which is the energy gap between split d-orbital energy levels caused by ligands interacting with a central metal atom. It provides information on how CFSE is calculated for octahedral and tetrahedral complexes, and factors that affect CFSE such as the nature of ligands and metal cation, complex geometry, and the metal's quantum number.Crystal field theory



Crystal field theoryShri Shankaracharya College, Bhilai,Junwani
Ėý
This document summarizes Crystal Field Theory, which considers the electrostatic interactions between metal ions and ligands. It describes ligands and metal ions as point charges that can have attractive or repulsive forces. This causes the d orbitals of the metal ion to split into two sets depending on if the field created by the ligands is weak or strong. The theory explains color in coordination compounds as being caused by d-d electron transitions under the influence of ligands. However, it has limitations like not accounting for other metal orbitals or the partial covalent nature of metal-ligand bonds.CRYSTAL FIELD THEORY OCTAHEDRAL SPLITTING.pptx



CRYSTAL FIELD THEORY OCTAHEDRAL SPLITTING.pptxMushiraBanu
Ėý
In coordination Chemistry, The CFT theory plays an important role... The splitting of Octahedral Complexes is neatly described in this presentation......The boron family



The boron familyJacob Adrian
Ėý
This document provides information about the boron family (Group 13) of the periodic table. It discusses the elements in Group 13 - boron (B), aluminium (Al), gallium (Ga), indium (In), and thallium (Tl). It details their electronic configurations, occurrence in nature, extraction methods, and chemical and physical properties. In particular, it focuses on the extraction of aluminium via the Bayer process and discusses the uses of aluminium and its environmental impacts.Jahn teller effect



Jahn teller effectMEGHNATH97
Ėý
This document discusses the Jahn-Teller effect, which states that any non-linear molecule in a degenerate electronic state will distort in order to remove that degeneracy. It provides background on the scientists Hermann Jahn and Edward Teller, who first identified this effect. The document then explains the two types of distortions that can occur - Z-out and Z-in - and provides examples of complexes that exhibit static and dynamic Jahn-Teller distortions. It concludes by stating that the Jahn-Teller effect removes degeneracy in complexes through elongation or compression and that elongation is more energetically favorable, resulting in more stable complexes.Electronic spectra of metal complexes-1



Electronic spectra of metal complexes-1SANTHANAM V
Ėý
This document discusses electronic spectra of metal complexes. It begins by relating the observed color of complexes to the light absorbed and corresponding wavelength ranges. It then discusses the use of electronic spectra to determine d-d transition energies and the factors that affect d orbital energies. Key terms like states, microstates, and quantum numbers are introduced. Configuration, inter-electronic repulsions described by Racah parameters, nephelauxetic effect, and spin-orbit coupling are explained as factors that determine the splitting of energy levels. Russell-Saunders and j-j coupling are outlined as approaches to describe spin-orbit interactions in light and heavy elements respectively.Crystal field theory 



Crystal field theory Shivaji Burungale
Ėý
This document discusses crystal field theory (CFT), which interprets the chemistry of coordination compounds. Some key points:
1. CFT was proposed by Hans Bethe in 1929 and modified by J.H. Van Vleck in 1935 to allow for some covalency. It assumes electrostatic interactions between metal ions and ligands.
2. In an octahedral crystal field, the d-orbitals split into two sets - the lower energy t2g orbitals and higher energy eg orbitals. The splitting is called the crystal field splitting parameter Îo.
3. The color of coordination compounds depends on the size of this splitting, as the energy difference corresponds to the absorption of photons.Lanthanide contraction



Lanthanide contractionNSSWAMIKARANAM
Ėý
LANTHANIDE CONTRACTION AND SEPARATION OF LANTHANIDES BY USING ION EXCHANGE METHOD AND SOLVENT EXTRACTION METHOD
Organometallic compounds



Organometallic compoundsArvind Singh Heer
Ėý
This document summarizes key concepts in organometallic chemistry. It discusses the definition of organometallic compounds as those containing metal-carbon bonds. It outlines different types of ligands that can bind to metals, including carbonyl, carbene, and cyclic Ï systems. It also describes principles for understanding bonding interactions between ligands and metals, such as the 18-electron rule and molecular orbital theory. Spectroscopic techniques for analyzing organometallic compounds are also summarized.Tanabe sugano diagram



Tanabe sugano diagramAfrina Jasy
Ėý
This document presents information on the Tanabe-Sugano diagram, which is used in coordination chemistry to predict absorptions in the UV-visible and IR spectra of coordination compounds. It was developed by Yukito Tanabe and Satoru Sugano in 1954 to explain the absorption spectra of octahedral complex ions. The diagram plots orbital energy as a function of the Racah parameter B versus the ligand field splitting parameter Îo/B. It can be used to determine the ordering of electronic states and predict possible electronic transitions based on parameters like Îo, Racah parameters B and C, symmetry rules, and term symbols of electronic configurations. The diagram has advantages over earlier Orgel diagrams in that it can be applied toCoordination chemistry - CFT



Coordination chemistry - CFTSANTHANAM V
Ėý
The document discusses Crystal Field Theory, which explains the bonding in transition metal complexes. It describes how the electrostatic interaction between ligand electrons and metal d-orbitals results in a splitting of the d-orbital energies. In an octahedral field, the t2g orbitals are stabilized more than the eg orbitals. Crystal Field Theory can explain properties like electronic spectra, magnetic moments, and color of complexes. The magnitude of splitting depends on factors like the metal ion, its charge, the ligands, and can be represented by the crystal field splitting energy Îo.Electronic spectra



Electronic spectramohammed rida
Ėý
This document discusses electronic spectra of metal complexes. It begins by defining quantum numbers related to electron configuration, such as L (total orbital angular momentum) and l (secondary quantum number). It then describes two main types of electronic transitions in coordination compounds: d-d transitions specific to metals, and charge-transfer transitions. The remainder of the document discusses charge-transfer transitions in more detail, defining ligand-to-metal and metal-to-ligand charge transfer, and how solvent polarity affects these transitions.Mo theory



Mo theorybapu thorat
Ėý
This document provides an overview of molecular orbital theory. It explains that molecular orbital theory describes molecules in terms of orbitals and electron configurations similar to atomic orbital theory. The key points are:
- Molecular orbitals are formed from the overlapping of atomic orbitals on different atoms.
- Bonding orbitals are formed from constructive interference and lower the energy, while antibonding orbitals are formed from destructive interference and increase the energy.
- Homonuclear diatomic molecules like H2, O2, and N2 are discussed as examples, with their molecular orbitals, bond orders, and magnetic properties explained.Molecular Orbital Theory



Molecular Orbital TheoryDr. Nandkishor Telkapalliwar
Ėý
- The document discusses molecular orbital theory, which describes chemical bonding through the combination of atomic orbitals into molecular orbitals.
- Key features include molecular orbitals being formed from linear combinations of atomic orbitals, with bonding, antibonding, and nonbonding molecular orbitals resulting. Electrons fill these orbitals based on orbital energy.
- The formation of molecular orbitals from atomic orbitals of hydrogen is used as an example, with bonding and antibonding molecular orbitals illustrated.Comaparative study of lanthanides and actinides



Comaparative study of lanthanides and actinidesRamyaR162
Ėý
Comparison of Lanthanides and Actinides. Points of Similarities and difference. Both show close resemblance because these involve filling of f-subshells. Both have coloured ions, low electronegativity, high reactivity and show magnetic properties.Orgel diagrams; D and F/P Orgel Diagrams 



Orgel diagrams; D and F/P Orgel Diagrams AafiaAslam
Ėý
Orgel diagrams depict the splitting of energy levels in transition metal complexes. They show the splitting of d electron configurations into terms based on whether the complex has an octahedral or tetrahedral ligand field. There are two main types of Orgel diagrams: D diagrams for d1, d4, d6, d9 complexes and F/P diagrams for d2, d3, d7, d8 complexes. The diagrams qualitatively show the possible electronic transitions between terms based on the complex's geometry and electron configuration. Orgel diagrams are useful for understanding the optical, magnetic, and spectral properties of transition metal complexes.Metal carbonyls



Metal carbonylssatyabrata sendh
Ėý
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. They were first synthesized in 1868 by passing carbon monoxide over platinum. Metal carbonyls typically obey the 18 electron rule and are often diamagnetic. They have applications as catalysts in organic synthesis and in producing pure metals like nickel. Precautions must be taken when using metal carbonyls due to their toxicity.Hard-Soft-Acids-and-Bases-HSAB.pptx



Hard-Soft-Acids-and-Bases-HSAB.pptxSATISH KOLA
Ėý
This document discusses Hard and Soft Acids and Bases (HSAB) theory presented by Dr. Satish S. Kola. It defines characteristics of hard vs soft acids and bases, with hard acids/bases being small with high oxidation states and no d-electrons, while soft acids/bases are large with low oxidation states and many d-electrons. Applications of HSAB principles are discussed, including predicting complex formation and metal catalyst poisoning. The theoretical basis involves concepts like pi-bonding, electrostatic interactions, and polarizability. Limitations are noted where inherent acid/base strength may override HSAB predictions.Term symbols



Term symbolsMithil Fal Desai
Ėý
Reference,
https://en.wikipedia.org/wiki/Term_symbol
James E. Huheey, Ellen A. Keiter, Richard L.Keiter and Okhil K. Medhi, Inorganic Chemistry, Principles of Structure and Reactivity. 4th Edn. Pearsons Lanthanide and actinide chemistry



Lanthanide and actinide chemistrySusovanBhowmik
Ėý
Lanthanide and Actinide Chemistry by Dr. Susovan Bhowmik,
Assistant Prof. Bankura Sammilani College, West BengalCrystal field stabilization energy



Crystal field stabilization energyDr. Krishna Swamy. G
Ėý
Factors affecting crystal field splitting and definition of CFSE and calculation of CFSE for octahedral and tetrahedral complexes Zero field splitting



Zero field splittingNaveed Bashir
Ėý
A ppt compiled by Yaseen Aziz Wani pursuing M.Sc Chemistry at University of Kashmir, J&K, India and Naveed Bashir Dar, a student of electrical engg. at NIT Srinagar.
Warm regards to Munnazir Bashir also for providing us with refreshing tea while we were compiling ppt.Rotational spectra 



Rotational spectra Rajal Pandya
Ėý
This Presentation gives the basic idea about Rotational Spectra a topic of Atomic and Molecular Spectroscopy in Modern Physics Coodination chemistry 



Coodination chemistry ushaSanmugaraj
Ėý
The document discusses valence bond theory and crystal field theory as they apply to coordination compounds.
Valence bond theory describes chemical bonding as occurring through the overlap of atomic orbitals. It can explain octahedral complexes through d2sp3 or sp3d2 hybridization of the metal's orbitals. Crystal field theory postulates that ligands exert an electrostatic field that splits the metal's d-orbitals into two energy levels. In an octahedral field, the eg orbitals have higher energy than the t2g orbitals. Complexes with weak ligands that cause little splitting tend to be high spin, while those with strong ligands that cause large splitting are low spin.Crystalfieldtheory 111204095834-phpapp01



Crystalfieldtheory 111204095834-phpapp01Liyana Jeinia
Ėý
The document discusses crystal field theory and its application to transition metal complexes. Crystal field theory explains the coloring of transition metal complexes based on the splitting of d-orbital energies caused by the electrostatic interaction between transition metal d-orbitals and ligand orbitals. Stronger field ligands cause more splitting and influence the complex's color and magnetic properties. Gemstone colors also result from transition metal ion impurities interacting with the crystal field of host minerals.More Related Content
What's hot (20)
Jahn teller effect



Jahn teller effectMEGHNATH97
Ėý
This document discusses the Jahn-Teller effect, which states that any non-linear molecule in a degenerate electronic state will distort in order to remove that degeneracy. It provides background on the scientists Hermann Jahn and Edward Teller, who first identified this effect. The document then explains the two types of distortions that can occur - Z-out and Z-in - and provides examples of complexes that exhibit static and dynamic Jahn-Teller distortions. It concludes by stating that the Jahn-Teller effect removes degeneracy in complexes through elongation or compression and that elongation is more energetically favorable, resulting in more stable complexes.Electronic spectra of metal complexes-1



Electronic spectra of metal complexes-1SANTHANAM V
Ėý
This document discusses electronic spectra of metal complexes. It begins by relating the observed color of complexes to the light absorbed and corresponding wavelength ranges. It then discusses the use of electronic spectra to determine d-d transition energies and the factors that affect d orbital energies. Key terms like states, microstates, and quantum numbers are introduced. Configuration, inter-electronic repulsions described by Racah parameters, nephelauxetic effect, and spin-orbit coupling are explained as factors that determine the splitting of energy levels. Russell-Saunders and j-j coupling are outlined as approaches to describe spin-orbit interactions in light and heavy elements respectively.Crystal field theory 



Crystal field theory Shivaji Burungale
Ėý
This document discusses crystal field theory (CFT), which interprets the chemistry of coordination compounds. Some key points:
1. CFT was proposed by Hans Bethe in 1929 and modified by J.H. Van Vleck in 1935 to allow for some covalency. It assumes electrostatic interactions between metal ions and ligands.
2. In an octahedral crystal field, the d-orbitals split into two sets - the lower energy t2g orbitals and higher energy eg orbitals. The splitting is called the crystal field splitting parameter Îo.
3. The color of coordination compounds depends on the size of this splitting, as the energy difference corresponds to the absorption of photons.Lanthanide contraction



Lanthanide contractionNSSWAMIKARANAM
Ėý
LANTHANIDE CONTRACTION AND SEPARATION OF LANTHANIDES BY USING ION EXCHANGE METHOD AND SOLVENT EXTRACTION METHOD
Organometallic compounds



Organometallic compoundsArvind Singh Heer
Ėý
This document summarizes key concepts in organometallic chemistry. It discusses the definition of organometallic compounds as those containing metal-carbon bonds. It outlines different types of ligands that can bind to metals, including carbonyl, carbene, and cyclic Ï systems. It also describes principles for understanding bonding interactions between ligands and metals, such as the 18-electron rule and molecular orbital theory. Spectroscopic techniques for analyzing organometallic compounds are also summarized.Tanabe sugano diagram



Tanabe sugano diagramAfrina Jasy
Ėý
This document presents information on the Tanabe-Sugano diagram, which is used in coordination chemistry to predict absorptions in the UV-visible and IR spectra of coordination compounds. It was developed by Yukito Tanabe and Satoru Sugano in 1954 to explain the absorption spectra of octahedral complex ions. The diagram plots orbital energy as a function of the Racah parameter B versus the ligand field splitting parameter Îo/B. It can be used to determine the ordering of electronic states and predict possible electronic transitions based on parameters like Îo, Racah parameters B and C, symmetry rules, and term symbols of electronic configurations. The diagram has advantages over earlier Orgel diagrams in that it can be applied toCoordination chemistry - CFT



Coordination chemistry - CFTSANTHANAM V
Ėý
The document discusses Crystal Field Theory, which explains the bonding in transition metal complexes. It describes how the electrostatic interaction between ligand electrons and metal d-orbitals results in a splitting of the d-orbital energies. In an octahedral field, the t2g orbitals are stabilized more than the eg orbitals. Crystal Field Theory can explain properties like electronic spectra, magnetic moments, and color of complexes. The magnitude of splitting depends on factors like the metal ion, its charge, the ligands, and can be represented by the crystal field splitting energy Îo.Electronic spectra



Electronic spectramohammed rida
Ėý
This document discusses electronic spectra of metal complexes. It begins by defining quantum numbers related to electron configuration, such as L (total orbital angular momentum) and l (secondary quantum number). It then describes two main types of electronic transitions in coordination compounds: d-d transitions specific to metals, and charge-transfer transitions. The remainder of the document discusses charge-transfer transitions in more detail, defining ligand-to-metal and metal-to-ligand charge transfer, and how solvent polarity affects these transitions.Mo theory



Mo theorybapu thorat
Ėý
This document provides an overview of molecular orbital theory. It explains that molecular orbital theory describes molecules in terms of orbitals and electron configurations similar to atomic orbital theory. The key points are:
- Molecular orbitals are formed from the overlapping of atomic orbitals on different atoms.
- Bonding orbitals are formed from constructive interference and lower the energy, while antibonding orbitals are formed from destructive interference and increase the energy.
- Homonuclear diatomic molecules like H2, O2, and N2 are discussed as examples, with their molecular orbitals, bond orders, and magnetic properties explained.Molecular Orbital Theory



Molecular Orbital TheoryDr. Nandkishor Telkapalliwar
Ėý
- The document discusses molecular orbital theory, which describes chemical bonding through the combination of atomic orbitals into molecular orbitals.
- Key features include molecular orbitals being formed from linear combinations of atomic orbitals, with bonding, antibonding, and nonbonding molecular orbitals resulting. Electrons fill these orbitals based on orbital energy.
- The formation of molecular orbitals from atomic orbitals of hydrogen is used as an example, with bonding and antibonding molecular orbitals illustrated.Comaparative study of lanthanides and actinides



Comaparative study of lanthanides and actinidesRamyaR162
Ėý
Comparison of Lanthanides and Actinides. Points of Similarities and difference. Both show close resemblance because these involve filling of f-subshells. Both have coloured ions, low electronegativity, high reactivity and show magnetic properties.Orgel diagrams; D and F/P Orgel Diagrams 



Orgel diagrams; D and F/P Orgel Diagrams AafiaAslam
Ėý
Orgel diagrams depict the splitting of energy levels in transition metal complexes. They show the splitting of d electron configurations into terms based on whether the complex has an octahedral or tetrahedral ligand field. There are two main types of Orgel diagrams: D diagrams for d1, d4, d6, d9 complexes and F/P diagrams for d2, d3, d7, d8 complexes. The diagrams qualitatively show the possible electronic transitions between terms based on the complex's geometry and electron configuration. Orgel diagrams are useful for understanding the optical, magnetic, and spectral properties of transition metal complexes.Metal carbonyls



Metal carbonylssatyabrata sendh
Ėý
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. They were first synthesized in 1868 by passing carbon monoxide over platinum. Metal carbonyls typically obey the 18 electron rule and are often diamagnetic. They have applications as catalysts in organic synthesis and in producing pure metals like nickel. Precautions must be taken when using metal carbonyls due to their toxicity.Hard-Soft-Acids-and-Bases-HSAB.pptx



Hard-Soft-Acids-and-Bases-HSAB.pptxSATISH KOLA
Ėý
This document discusses Hard and Soft Acids and Bases (HSAB) theory presented by Dr. Satish S. Kola. It defines characteristics of hard vs soft acids and bases, with hard acids/bases being small with high oxidation states and no d-electrons, while soft acids/bases are large with low oxidation states and many d-electrons. Applications of HSAB principles are discussed, including predicting complex formation and metal catalyst poisoning. The theoretical basis involves concepts like pi-bonding, electrostatic interactions, and polarizability. Limitations are noted where inherent acid/base strength may override HSAB predictions.Term symbols



Term symbolsMithil Fal Desai
Ėý
Reference,
https://en.wikipedia.org/wiki/Term_symbol
James E. Huheey, Ellen A. Keiter, Richard L.Keiter and Okhil K. Medhi, Inorganic Chemistry, Principles of Structure and Reactivity. 4th Edn. Pearsons Lanthanide and actinide chemistry



Lanthanide and actinide chemistrySusovanBhowmik
Ėý
Lanthanide and Actinide Chemistry by Dr. Susovan Bhowmik,
Assistant Prof. Bankura Sammilani College, West BengalCrystal field stabilization energy



Crystal field stabilization energyDr. Krishna Swamy. G
Ėý
Factors affecting crystal field splitting and definition of CFSE and calculation of CFSE for octahedral and tetrahedral complexes Zero field splitting



Zero field splittingNaveed Bashir
Ėý
A ppt compiled by Yaseen Aziz Wani pursuing M.Sc Chemistry at University of Kashmir, J&K, India and Naveed Bashir Dar, a student of electrical engg. at NIT Srinagar.
Warm regards to Munnazir Bashir also for providing us with refreshing tea while we were compiling ppt.Rotational spectra 



Rotational spectra Rajal Pandya
Ėý
This Presentation gives the basic idea about Rotational Spectra a topic of Atomic and Molecular Spectroscopy in Modern Physics Coodination chemistry 



Coodination chemistry ushaSanmugaraj
Ėý
The document discusses valence bond theory and crystal field theory as they apply to coordination compounds.
Valence bond theory describes chemical bonding as occurring through the overlap of atomic orbitals. It can explain octahedral complexes through d2sp3 or sp3d2 hybridization of the metal's orbitals. Crystal field theory postulates that ligands exert an electrostatic field that splits the metal's d-orbitals into two energy levels. In an octahedral field, the eg orbitals have higher energy than the t2g orbitals. Complexes with weak ligands that cause little splitting tend to be high spin, while those with strong ligands that cause large splitting are low spin.Similar to Crystal field theory (20)
Crystalfieldtheory 111204095834-phpapp01



Crystalfieldtheory 111204095834-phpapp01Liyana Jeinia
Ėý
The document discusses crystal field theory and its application to transition metal complexes. Crystal field theory explains the coloring of transition metal complexes based on the splitting of d-orbital energies caused by the electrostatic interaction between transition metal d-orbitals and ligand orbitals. Stronger field ligands cause more splitting and influence the complex's color and magnetic properties. Gemstone colors also result from transition metal ion impurities interacting with the crystal field of host minerals.Warna & kemagnetan senyawa kompleks 2017 1



Warna & kemagnetan senyawa kompleks 2017 1AyumaGanbatte AlKaoru
Ėý
The document discusses electronic spectra and color of transition metal complexes. It explains that the color of complexes is due to electronic transitions between split d-orbital energy levels of the metal ion. Crystal field theory is used to describe the splitting of d-orbitals in an octahedral ligand field, which determines the color. Complexes with strong field ligands have large splitting and absorb at higher energies, appearing more intensely colored.Ų
ØاØķØąØ§ØŠŲŲŲ
ŲØ§ØĄ-ØŠŲاØģŲŲØĐ-اŲŲ
ØąØŲØĐ-اŲØŦاŲØŦØĐ-ŲØĩŲ-اŲŲ.ąčąčģŲģæ



Ų
ØاØķØąØ§ØŠŲŲŲ
ŲØ§ØĄ-ØŠŲاØģŲŲØĐ-اŲŲ
ØąØŲØĐ-اŲØŦاŲØŦØĐ-ŲØĩŲ-اŲŲ.ąčąčģŲģæRiandyPutra1
Ėý
This document provides an overview of transition metal coordination chemistry. It discusses the following key points in 3 sentences or less:
Metal complexes consist of a central metal ion bonded to surrounding ligand molecules or ions. The ligands donate lone pairs of electrons to form coordinate covalent bonds with the metal. The geometry and electronic structure of complexes is influenced by the coordination number, ligands present, and hybridization state of the metal ion.D and f block



D and f blockRohitPatil549
Ėý
The document discusses the d-block and f-block elements of the periodic table. It provides details about their electronic configurations, properties, and reactions. The d-block contains the transition metals whose d orbitals are filled from groups 3 to 12. The f-block contains the inner transition metals whose 4f and 5f orbitals are filled. Elements in these blocks can exhibit a variety of oxidation states and form complexes due to their electronic structure.d and f block elements.pptx



d and f block elements.pptxPariJain51
Ėý
The document discusses d-block and f-block elements. It provides information on:
1. The d-block elements have incompletely filled d orbitals and include elements from groups 3 to 12 in the periodic table.
2. Transition metals show variable oxidation states due to their ability to gain or lose ns and (n-1)d electrons. They form colored compounds and complexes due to their unpaired d electrons.
3. The f-block elements have incompletely filled 4f and 5f orbitals and include the lanthanides and actinides which follow lanthanum and actinium respectively.topic_13_powerpoint-converted.pptx



topic_13_powerpoint-converted.pptxJaimin Surani
Ėý
This document describes the properties of transition elements and their ions. Transition elements have variable oxidation states because their 4s and 3d orbitals are close in energy. They form colored complexes due to d-d transitions absorbing visible light. The color depends on the identity and oxidation state of the metal ion and the ligands present, which influence the splitting of d orbitals. Transition metals also show paramagnetism and some are ferromagnetic due to unpaired d electrons.Why Gemstones have multiple Colors___Introduction of Crystal Field Theory & M...



Why Gemstones have multiple Colors___Introduction of Crystal Field Theory & M...Heman Chen
Ėý
Gemstones aren't just pretty; they're a science lesson! Colors in gems come from how transition metal ions play with light. Think of it like a dance: ions and ligands groove in a crystal field, splitting energy levels and creating a colorful show. The separation energy, or âģ, is the DJ that controls the party's vibe. Different fields, like square or octahedral, have their own unique beats.
Molecular Orbital Theory adds another layer, with charge transfers and covalent bonds creating color magic. It's like a power duo in a band, where one member (the metal) passes the mic (electrons) to another (the ligand), and voilà , a new color is born.
Energy Band Theory talks about how electrons move in a solid, forming bands that determine if a material conducts or not. It's like a traffic system for electrons, with band gaps being the toll booths.
Physical optical effects, like dispersion and interference, add the final touches to a gem's color palette. It's the gem's structure and properties that paint the picture.INORGANIC CHEMISTRY 1.2-TRANSITION ELEMENT



INORGANIC CHEMISTRY 1.2-TRANSITION ELEMENTshahzadebaujiti
Ėý
This document discusses transition elements and their properties. Transition elements are elements that have incompletely filled d orbitals and intermediate properties between s-block and p-block elements. There are three series of transition metals. The document focuses on the transition metals of the first series, which have at least one unpaired electron in the 3d subshell. It discusses their electronic configurations and provides examples. The document also discusses general properties of transition metals like color formation, variable oxidation states, and ability to form complex compounds. It explains color formation using crystal field theory and the splitting of d orbitals by ligands. Finally, it discusses the basics of complex compound formation including central atoms, ligands, and naming conventions.CFT 1.pptx



CFT 1.pptxMdSohaib7
Ėý
Crystal field theory (CFT) explains the splitting of d-orbital energies when a metal ion is placed in a ligand field. For an octahedral complex, the d-orbitals split into a lower-energy t2g set and a higher-energy eg set. The extent of this splitting, known as the crystal field splitting energy (CFSE), depends on factors like the nature of ligands. CFT can be used to understand properties like ionic radii variations, hydration enthalpies, and ligand field strengths. Tetrahedral complexes have a smaller CFSE than octahedral complexes.Coordination Compounds CLASS 12 CHEMISTRY



Coordination Compounds CLASS 12 CHEMISTRYSimonRiley43
Ėý
Coordination Compounds CLASS 12 CHEMISTRY CBSE NCERTCo ordination compounds



Co ordination compoundsrishiram mahato
Ėý
This document discusses coordination compounds and provides several examples. It begins with an introduction to coordination compounds, which involve coordinate covalent bonds between ligands and a central atom. Some early and well-known coordination complexes are mentioned. The document then discusses the occurrence of coordination compounds such as chlorophyll, hemoglobin, and cytochromes. It also briefly describes the mineral fluorite. The document concludes by discussing the properties and applications of coordination compounds, specifically mentioning the chemotherapy drug cisplatin.Co ordination compounds



Co ordination compoundsrishiram mahato
Ėý
This document discusses coordination compounds and provides several examples. It begins with an introduction to coordination compounds, which involve coordinate covalent bonds between ligands and a central atom. Some early and well-known coordination complexes are mentioned. The document then discusses the occurrence of coordination compounds such as chlorophyll, hemoglobin, and cytochromes. It also briefly describes the mineral fluorite. The document concludes by discussing the properties and applications of coordination compounds, specifically mentioning the chemotherapy drug cisplatin.Non bonding electrons



Non bonding electronsPicasa_10
Ėý
It contains information about various theories of chemical bonding, mainly CFT. It discusses the splitting diagrams of octahedral, tetrahedral and square planar fields. Jahn-Teller distortion is also explained here in simple terms.Bonding in Coordination Compounds



Bonding in Coordination CompoundsChris Sonntag
Ėý
How do we describe the bonding between transition metal (ions) and their ligands (like water, ammonia, CO etc) ?
The Crystal Field Model gives a simple theory to explain electronic spectra.Coordination compounds



Coordination compoundsDr Robert Craig PhD
Ėý
This document discusses coordination compounds, which are metal ions surrounded by ligands. Common ligands include chloride, cyanide, ammonia, and ethylenediamine. Coordination compounds play important roles in metal purification and identification. Ethylenediaminetetraacetate (EDTA) forms soluble complexes that can be used to treat heavy metal poisoning. Ligands are classified as monodentate, bidentate, or polydentate based on the number of donor atoms. Crystal field theory explains the colors of coordination compounds based on splitting of the metal's d-orbitals. Ligands can be ranked in a spectrochemical series based on their field strength. Experiments are described to observe the colors and absorptioncry



cryBhawana Saklani
Ėý
Crystal field theory was proposed in the 1950s to describe the bonding in ionic crystals and metal complexes. It uses an electrostatic model to explain how ligands interact with the d-orbitals of a central metal ion. This interaction splits the degeneracy of the d-orbitals into lower-energy orbitals (t2g) and higher-energy orbitals (eg). The crystal field splitting energy is determined by factors like the ligand type, metal oxidation state, and complex geometry. Crystal field theory can be used to determine properties of complexes such as color, magnetism, and spinel structures. It provides explanations for phenomena like Jahn-Teller distortions but has limitations and cannot fully describe covalent bonding.Inorganic materials Part 2/2



Inorganic materials Part 2/2Chris Sonntag
Ėý
(1) The document discusses doping of semiconductors and transition metal oxides, including n-type and p-type doping of silicon. It also covers band structure diagrams and density of states plots.
(2) Preparation methods for metal oxides include molecular synthesis and solid state synthesis. Modification of solids can occur through ion exchange or intercalation. Lithium ion batteries operate through lithium intercalation into graphite.
(3) Characterization techniques covered are XRD for crystal structure analysis and electron microscopy. Magnetic properties depend on temperature; ferromagnets become paramagnetic above the Curie temperature. Spinels can exhibit ferrimagnetism from opposing sublatticeB.sc II chemistry of transitional elements iv



B.sc II chemistry of transitional elements ivDept of chemistry,Shri Shivaji Science College,Amravati
Ėý
This document discusses transition metal coordination compounds and their properties. It begins by explaining color and absorption spectra in relation to an artist's color wheel. It then discusses crystal field theory and how ligand bonding splits the d-orbital energies of metal ions. Stronger field ligands create more splitting. This splitting determines the wavelengths absorbed and the observed color. Examples are given of color changes from oxidation state or ligand changes. The spectrochemical series ranks ligands by field strength. Magnetic properties depend on unpaired electrons. Complexes can be high-spin or low-spin depending on the relative energies of pairing and splitting. Examples of splitting patterns and orbital occupancy are shown for octahedral, tetrahedral, and square planar complexes. Hemoglobin and carbonŲ
ØاØķØąØ§ØŠŲŲŲ
ŲØ§ØĄ-ØŠŲاØģŲŲØĐ-اŲŲ
ØąØŲØĐ-اŲØŦاŲØŦØĐ-ŲØĩŲ-اŲŲ.ąčąčģŲģæ



Ų
ØاØķØąØ§ØŠŲŲŲ
ŲØ§ØĄ-ØŠŲاØģŲŲØĐ-اŲŲ
ØąØŲØĐ-اŲØŦاŲØŦØĐ-ŲØĩŲ-اŲŲ.ąčąčģŲģæRiandyPutra1
Ėý
B.sc II chemistry of transitional elements iv



B.sc II chemistry of transitional elements ivDept of chemistry,Shri Shivaji Science College,Amravati
Ėý
Recently uploaded (20)
APM People Interest Network Conference - Oliver Randall & David Bovis - Own Y...



APM People Interest Network Conference - Oliver Randall & David Bovis - Own Y...Association for Project Management
Ėý
APM People Interest Network Conference 2025
- Autonomy, Teams and Tension
- Oliver Randall & David Bovis
- Own Your Autonomy
Oliver Randall
Consultant, Tribe365
Oliver is a career project professional since 2011 and started volunteering with APM in 2016 and has since chaired the People Interest Network and the North East Regional Network. Oliver has been consulting in culture, leadership and behaviours since 2019 and co-developed HPTMÂŪâŊan off the shelf high performance framework for teams and organisations and is currently working with SAS (Stellenbosch Academy for Sport) developing the culture, leadership and behaviours framework for future elite sportspeople whilst also holding down work as a project manager in the NHS at North Tees and Hartlepool Foundation Trust.
David Bovis
Consultant, Duxinaroe
A Leadership and Culture Change expert, David is the originator of BTFAâĒ and The Dux Model.
With a Masters in Applied Neuroscience from the Institute of Organisational Neuroscience, he is widely regarded as the âGo-Toâ expert in the field, recognised as an inspiring keynote speaker and change strategist.
He has an industrial engineering background, majoring in TPS / Lean. David worked his way up from his apprenticeship to earn his seat at the C-suite table. His career spans several industries, including Automotive, Aerospace, Defence, Space, Heavy Industries and Elec-Mech / polymer contract manufacture.
Published in Londonâs Evening Standard quarterly business supplement, James Caanâs âYour businessâ Magazine, âQuality Worldâ, the Lean Management Journal and Cambridge Universities âPMAâ, he works as comfortably with leaders from FTSE and Fortune 100 companies as he does owner-managers in SMEâs. He is passionate about helping leaders understand the neurological root cause of a high-performance culture and sustainable change, in business.
Session | Own Your Autonomy â The Importance of Autonomy in Project Management
#OwnYourAutonomy is aiming to be a global APM initiative to position everyone to take a more conscious role in their decision making process leading to increased outcomes for everyone and contribute to âa world in which all projects succeedâ.
We want everyone to join the journey.
#OwnYourAutonomy is the culmination of 3 years of collaborative exploration within the Leadership Focus Group which is part of the APM People Interest Network. The work has been pulled together using the 5 HPTMÂŪ Systems and the BTFA neuroscience leadership programme.
https://www.linkedin.com/showcase/apm-people-network/about/Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ėý
Finals of Rass MELAI : a Music, Entertainment, Literature, Arts and Internet Culture Quiz organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. Digital Tools with AI for e-Content Development.pptx



Digital Tools with AI for e-Content Development.pptxDr. Sarita Anand
Ėý
This ppt is useful for not only for B.Ed., M.Ed., M.A. (Education) or any other PG level students or Ph.D. scholars but also for the school, college and university teachers who are interested to prepare an e-content with AI for their students and others.SOCIAL CHANGE(a change in the institutional and normative structure of societ...



SOCIAL CHANGE(a change in the institutional and normative structure of societ...DrNidhiAgarwal
Ėý
This PPT is showing the effect of social changes in human life and it is very understandable to the students with easy language.in this contents are Itroduction, definition,Factors affecting social changes ,Main technological factors, Social change and stress , what is eustress and how social changes give impact of the human's life.Database population in Odoo 18 - Odoo slides



Database population in Odoo 18 - Odoo slidesCeline George
Ėý
In this slide, weâll discuss the database population in Odoo 18. In Odoo, performance analysis of the source code is more important. Database population is one of the methods used to analyze the performance of our code. N.C. DPI's 2023 Language Diversity Briefing



N.C. DPI's 2023 Language Diversity BriefingMebane Rash
Ėý
The number of languages spoken in NC public schools.Essentials of a Good PMO, presented by Aalok Sonawala



Essentials of a Good PMO, presented by Aalok SonawalaAssociation for Project Management
Ėý
APM event hosted by the South Wales and West of England Network (SWWE Network)
Speaker: Aalok Sonawala
The SWWE Regional Network were very pleased to welcome Aalok Sonawala, Head of PMO, National Programmes, Rider Levett Bucknall on 26 February, to BAWA for our first face to face event of 2025. Aalok is a member of APMâs Thames Valley Regional Network and also speaks to members of APMâs PMO Interest Network, which aims to facilitate collaboration and learning, offer unbiased advice and guidance.
Tonight, Aalok planned to discuss the importance of a PMO within project-based organisations, the different types of PMO and their key elements, PMO governance and centres of excellence.
PMOâs within an organisation can be centralised, hub and spoke with a central PMO with satellite PMOs globally, or embedded within projects. The appropriate structure will be determined by the specific business needs of the organisation. The PMO sits above PM delivery and the supply chain delivery teams.
For further information about the event please click here.A PPT Presentation on The Princess and the God: A tale of ancient India by A...



A PPT Presentation on The Princess and the God: A tale of ancient India by A...Beena E S
Ėý
A PPT Presentation on The Princess and the God: A tale of ancient India by Aaron ShepardKaun TALHA quiz Prelims - El Dorado 2025



Kaun TALHA quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ėý
Prelims of Kaun TALHA : a Travel, Architecture, Lifestyle, Heritage and Activism quiz, organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. How to attach file using upload button Odoo 18



How to attach file using upload button Odoo 18Celine George
Ėý
In this slide, weâll discuss on how to attach file using upload button Odoo 18. Odoo features a dedicated model, 'ir.attachments,' designed for storing attachments submitted by end users. We can see the process of utilizing the 'ir.attachments' model to enable file uploads through web forms in this slide.Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ėý
Prelims of Rass MELAI : a Music, Entertainment, Literature, Arts and Internet Culture Quiz organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. Information Technology for class X CBSE skill Subject



Information Technology for class X CBSE skill SubjectVEENAKSHI PATHAK
Ėý
These questions are based on cbse booklet for 10th class information technology subject code 402. these questions are sufficient for exam for first lesion. This subject give benefit to students and good marks. if any student weak in one main subject it can replace with these marks.How to Configure Flexible Working Schedule in Odoo 18 Employee



How to Configure Flexible Working Schedule in Odoo 18 EmployeeCeline George
Ėý
In this slide, weâll discuss on how to configure flexible working schedule in Odoo 18 Employee module. In Odoo 18, the Employee module offers powerful tools to configure and manage flexible working schedules tailored to your organization's needs.APM People Interest Network Conference - Oliver Randall & David Bovis - Own Y...



APM People Interest Network Conference - Oliver Randall & David Bovis - Own Y...Association for Project Management
Ėý
Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ėý
Kaun TALHA quiz Prelims - El Dorado 2025



Kaun TALHA quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ėý
Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ėý
Crystal field theory
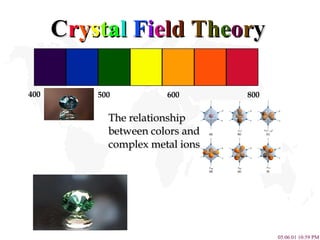
- 1. C r y s t a l F i e l d T h e o r y The relationship between colors and complex metal ions 400 500 600 800
- 2. Transition Metal Gems Gemstone owe their color from trace transition-metal ions Corundum mineral, Al 2 O 3 : Colorless Cr ï§ Al : Ruby Mn ï§ Al: Amethyst Fe ï§ Al: Topaz Ti &Co ï§ Al: Sapphire Beryl mineral, Be 3 Al 2 Si 6 O 18 : Colorless Cr ï§ Al : Emerald Fe ï§ Al : Aquamarine
- 3. Crystal-Field Theory Model explaining bonding for transition metal complexes âĒ Originally developed to explain properties for crystalline material âĒ Basic idea: Electrostatic interaction between lone-pair electrons result in coordination.
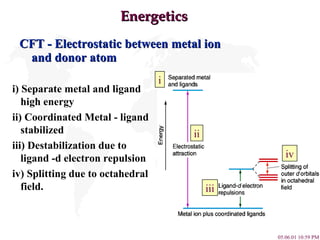
- 4. Energetics CFT - Electrostatic between metal ion and donor atom i) Separate metal and ligand high energy ii) Coordinated Metal - ligand stabilized iii) Destabilization due to ligand -d electron repulsion iv) Splitting due to octahedral field. i ii iii iv
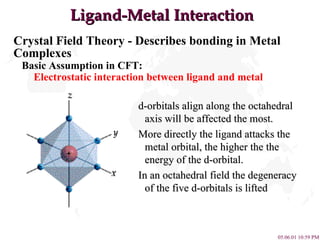
- 5. Ligand-Metal Interaction Crystal Field Theory - Describes bonding in Metal Complexes Basic Assumption in CFT: Electrostatic interaction between ligand and metal d-orbitals align along the octahedral axis will be affected the most. More directly the ligand attacks the metal orbital, the higher the the energy of the d-orbital. In an octahedral field the degeneracy of the five d-orbitals is lifted
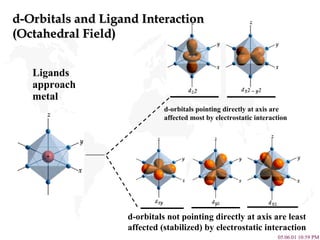
- 6. d-Orbitals and Ligand Interaction (Octahedral Field) Ligands approach metal d-orbitals not pointing directly at axis are least affected (stabilized) by electrostatic interaction d-orbitals pointing directly at axis are affected most by electrostatic interaction
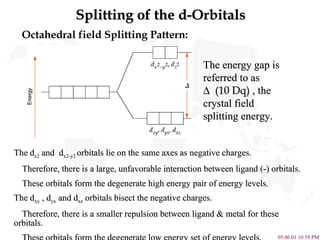
- 7. Splitting of the d-Orbitals Octahedral field Splitting Pattern: The energy gap is referred to as ïï ï (10 Dq) , the crystal field splitting energy. The d z2 and d x2-y2 orbitals lie on the same axes as negative charges. Therefore, there is a large, unfavorable interaction between ligand (-) orbitals. These orbitals form the degenerate high energy pair of energy levels. The d xy , d yx and d xz orbitals bisect the negative charges. Therefore, there is a smaller repulsion between ligand & metal for these orbitals. These orbitals form the degenerate low energy set of energy levels.
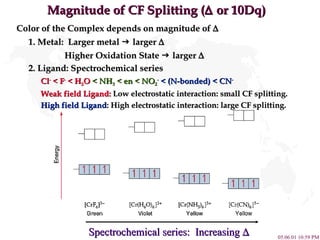
- 8. Magnitude of CF Splitting ( ï or 10Dq) Color of the Complex depends on magnitude of ï 1. Metal: Larger metal ï§ larger ï Higher Oxidation State ï§ larger ï 2. Ligand: Spectrochemical series Cl - < F - < H 2 O < NH 3 < en < NO 2 - < (N-bonded) < CN - Weak field Ligand: Low electrostatic interaction: small CF splitting. High field Ligand : High electrostatic interaction: large CF splitting. Spectrochemical series: Increasing ï
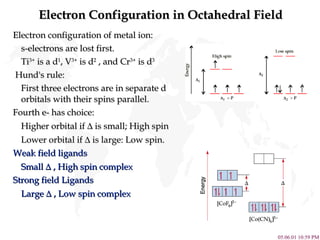
- 9. Electron Configuration in Octahedral Field Electron configuration of metal ion: s-electrons are lost first. Ti 3+ is a d 1 , V 3+ is d 2 , and Cr 3+ is d 3 Hund's rule: First three electrons are in separate d orbitals with their spins parallel. Fourth e- has choice: Higher orbital if ï is small; High spin Lower orbital if ï is large: Low spin. Weak field ligands Small ï , High spin complex Strong field Ligands Large ï , Low spin complex
- 10. High Spin Vs. Low Spin (d 1 to d 10 ) Electron Configuration for Octahedral complexes of metal ion having d 1 to d 10 configuration [M(H 2 O) 6 ] +n . Only the d 4 through d 7 cases have both high-spin and low spin configuration . Electron configurations for octahedral complexes of metal ions having from d 1 to d 10 configurations. Only the d 4 through d 7 cases have both high-spin and low-spin configurations.
- 11. Color Absorption of Co 3+ Complexes The Colors of Some Complexes of the Co 3+ Ion The complex with fluoride ion, [CoF 6 ] 3+ , is high spin and has one absorption band. The other complexes are low spin and have two absorption bands. In all but one case, one of these absorptionsis in the visible region of the spectrum. The wavelengths refer to the center of that absorption band. Complex Ion Wavelength of Color of Light Color of Complex light absorbed Absorbed [CoF 6 ] 3+ 700 (nm) Red Green [Co(C 2 O 4 ) 3 ] 3+ 600, 420 Yellow, violet Dark green [Co(H 2 O) 6 ] 3+ 600, 400 Yellow, violet Blue-green [Co(NH 3 ) 6 ] 3+ 475, 340 Blue, violet Yellow-orange [Co(en) 3 ] 3+ 470, 340 Blue, ultraviolet Yellow-orange [Co(CN) 6 ] 3+ 310 Ultraviolet Pale Yellow
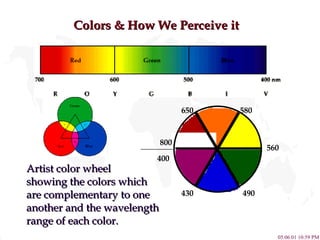
- 12. Colors & How We Perceive it 800 430 650 580 560 490 Artist color wheel showing the colors which are complementary to one another and the wavelength range of each color. 400
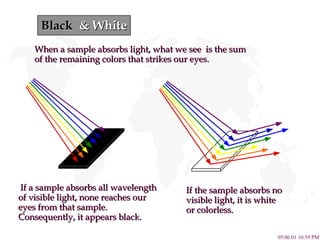
- 13. Black & White If a sample absorbs all wavelength of visible light, none reaches our eyes from that sample. Consequently, it appears black. When a sample absorbs light, what we see is the sum of the remaining colors that strikes our eyes. If the sample absorbs no visible light, it is white or colorless.
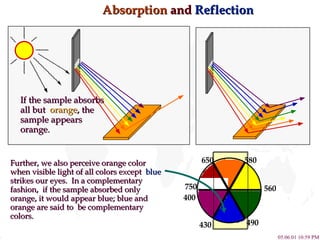
- 14. Absorption and Reflection If the sample absorbs all but orange , the sample appears orange. Further, we also perceive orange color when visible light of all colors except blue strikes our eyes. In a complementary fashion, if the sample absorbed only orange, it would appear blue; blue and orange are said to be complementary colors. 750 430 650 580 560 490 400
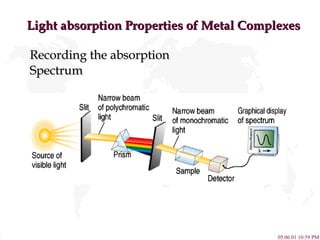
- 15. Light absorption Properties of Metal Complexes Recording the absorption Spectrum
- 16. Complex Influence on Color Compounds of Transition metal complexes solution. [Fe(H 2 O) 6 ] 3+ [Co(H 2 O) 6 ] 2+ [Ni(H 2 O) 6 ] 2+ [Cu(H 2 O) 6 ] 2+ [Zn(H 2 O) 6 ] 2+ 800 430 650 580 560 490 400
- 17. Color Absorption of Co 3+ Complexes The Colors of Some Complexes of the Co 3+ Ion The complex with fluoride ion, [CoF 6 ] 3+ , is high spin and has one absorption band. The other complexes are low spin and have two absorption bands. In all but one case, one of these absorptionsis in the visible region of the spectrum. The wavelengths refer to the center of that absorption band.
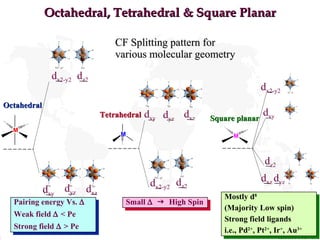
- 18. Octahedral, Tetrahedral & Square Planar CF Splitting pattern for various molecular geometry Octahedral Tetrahedral Square planar Pairing energy Vs. ï Weak field ï < Pe Strong field ï > Pe Small ï ï§ High Spin Mostly d 8 (Majority Low spin) Strong field ligands i.e., Pd 2+ , Pt 2+ , Ir + , Au 3+ d z2 d x2-y2 d xz d xy d yz d x2-y2 d z2 d xz d xy d yz d xz d z2 d x2-y2 d xy d yz
- 19. Summary Crystal Field Theory provides a basis for explaining many features of transition-metal complexes. Examples include why transition metal complexes are highly colored, and why some are paramagnetic while others are diamagnetic. The spectrochemical series for ligands explains nicely the origin of color and magnetism for these compounds. There is evidence to suggest that the metal-ligand bond has covalent character which explains why these complexes are very stable. Molecular Orbital Theory can also be used to describe the bonding scheme in these complexes. A more in depth analysis is required however.








