Formula fizik f4
- 1. BAB 1 : PENGENALAN KEPADA FIZIK Kuantiti Asas Kuantiti Terbitan Panjang m Isipadu m3 Jisim kg Ketumpatan kgm-3 Masa s Halaju ms-1 Arus Elektrik A Pecutan ms-2 Suhu K Daya kgms-2 Tenaga kgm2 s-2 Kerja kgm2 s-2 Momentum kgms-1 BAB 2 : DAYA DAN GERAKAN Formula Asas : Gerakan Linear  a = (v-u)/t  s = ut + (1/2)at2  v2 = u2 + 2as Keabadian momentum : m1u1 + m2u2 = m1v1 + m2v2 Daya, F = ma (N) Impuls, Ft = mv – mu Daya impuls, F = (mv – mu)/ t Berat, W = mg Kerja , W = F x s Tenaga keupayaan gravity,Ep = mgh Tenaga kinetic, Ek= (1/2)mv2 Kuasa, P = W/t (Watt) Hukum Hook (spring) F = kx Tenaga keupayaan kenyal, Ep = (1/2)kx2 = (1/2)Fx BAB 3: DAYA DAN TEKANAN Tekanan, P = F/A Tekanan dalam cecair, P = hpg  h = ketinggian turus cecair  p = ketumpatan bendalir  g = pecutan graviti ( 10ms-2 ) Tekanan atmosfera, Patm = 1 Atm = 76cmhg = 101.3 kNm-2 = 101.3 kPa Semakin bertambah altitud (ketinggian) ,semakin berkurang tekan atmosfera Patm Prinsip Pascal Apabila tekanan dikenakan kepada bendalir yang tertutup , tekanan akan dihantar sama sepanjang laluan cecair tersebut F1 = F2 A1 A2 atau A1d1 = A2d2 Prinsip Archimedes Apabila sesuatu objek tenggelam sebahagian atau sepenuhnya di dalam bendalir (air) , berat cecair yang disesarkan adalah sama dengan daya julangan objek tersebut.
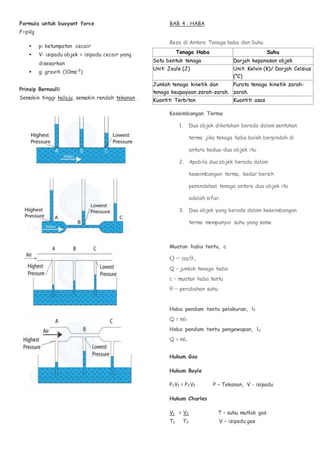
- 2. Formula untuk buoyant force F=pVg  p: ketumpatan cecair  V: isipadu objek = isipadu cecair yang disesarkan  g: graviti (10ms-2 ) Prinsip Bernoulli Semakin tinggi halaju, semakin rendah tekanan . BAB 4 : HABA Beza di Antara Tenaga haba dan Suhu Keseimbangan Terma 1. Dua objek dikatakan berada dalam sentuhan terma jika tenaga haba boleh berpindah di antara kedua-dua objek itu. 2. Apabila dua objek berada dalam keseimbangan terma, kadar bersih pemindahan tenaga antara dua objek itu adalah sifar. 3. Dua objek yang berada dalam keseimbangan terma mempunyai suhu yang sama Muatan haba tentu, c Q = mcθ , Q – jumlah tenaga haba c – muatan haba tentu θ – perubahan suhu Haba pendam tentu pelakuran, lf Q = mlf Haba pendam tentu pengewapan, lv Q = mlv Hukum Gas Hukum Boyle P1V1 = P2V2 P – Tekanan, V - isipadu Hukum Charles V1 = V2 T – suhu mutlak gas T1 T2 V – isipadu gas Tenaga Haba Suhu Satu bentuk tenaga Darjah kepanasan objek Unit: Joule (J) Unit: Kelvin (K)/ Darjah Celsius (°C) Jumlah tenaga kinetik dan tenaga keupayaan zarah-zarah. Purata tenaga kinetik zarah- zarah. Kuantiti Terbitan Kuantiti asas
- 3. Hukum Tekanan P1 = P2 P - tekanan T1 T2 T – suhu mutlak BAB 5 : CAHAYA Pantulan cahaya Imej dalam cermin satah Hukum snell n = sin i sin r n = indeks biasan, i – sudut tuju, r – sudut biasan Indeks biasan = laju cahaya dalam vakum laju cahaya dalam satu medium atau n = c v Dalam Nyata dan Dalam Ketara Indeks biasan = dalam nyata dalam ketara atau n = D d Pantulan dalam penuh dan sudut genting n = 1 sin c c – sudut genting Persamaan kanta 1 + 1 = 1 u v f u – jarak objek, v – jarak imej, f – panjang focus Pembesaran, m m = saiz imej, v saiz objek, u