Ib quan chemistry(mole concept)
Download as pptx, pdf4 likes2,542 views
1. The relative formula mass of calcium carbonate (CaCO3) is 100 g/mol. 2. One mole of calcium carbonate will react with 2 moles of hydrochloric acid (HCl). 3. Therefore, the mass of hydrochloric acid that will react with 1 mole (100 g) of calcium carbonate is 2 x 36.5 g = 73 g, since the molar mass of HCl is 36.5 g/mol.
1 of 16
Downloaded 69 times






Ad
Recommended
energy from the volcano.pptx
energy from the volcano.pptxCYRILCONSTANTINO2
╠²
Geothermal energy is generated from the heat within the Earth's interior. Geothermal power plants tap into this heat by drilling wells deep underground to access steam or hot water, with plants often located near volcanoes. When the hot water reaches the surface, the decrease in pressure causes it to turn to steam to spin turbines and generate electricity. The Philippines ranks second worldwide in geothermal energy production, with 27% of its electricity coming from geothermal power plants.Measuring mass
Measuring massLakshmy Subramaniam
╠²
Mass is the amount of matter in an object, while weight depends on both mass and gravity. Common tools for measuring mass include triple beam balances and beam balances. The triple beam balance uses sliding weights to balance the mass of an object, while the beam balance uses a central beam to measure mass. Mass is quantified using metric units like grams and kilograms, with the kilogram being the SI base unit of mass. Weight varies with changes in gravity, while mass remains constant regardless of location.Money in UAE for pre-school
Money in UAE for pre-schoolGloria Mazhim De Decker
╠²
The document introduces preschoolers to currency in Dubai, specifically focusing on the 1 dirham coin and the 5 and 10 dirham notes. It provides simple exercises on how many coins and notes are needed to make specific amounts. The overall purpose is to help children start learning about saving money.Algebra 1 unit 1.1
Algebra 1 unit 1.1Mark Ryder
╠²
The document discusses mathematical expressions and operations such as:
- Area is calculated as length times width
- Variables such as A, L, and W represent unknown numbers
- Expressions contain numbers, variables, and arithmetic operations
- Multiplication can be shown with dots or parentheses
- Terms like sum, difference, product, and quotient are used for addition, subtraction, multiplication, and division1.basic of fractions
1.basic of fractionsDreams4school
╠²
This document provides instructions on how to change whole numbers to fractions, add and subtract fractions, and multiply and divide fractions. It begins by explaining how to write a whole number as a fraction by multiplying the whole number by the denominator. It then discusses reducing fractions to lower or lowest terms through dividing the numerator and denominator by common factors. The document also covers finding the least common denominator to add or subtract fractions, and how to add and subtract mixed numbers by first handling the whole numbers and then the fractions. It concludes with an overview of multiplying fractions by multiplying the numerators and denominators, and dividing fractions by keeping the first fraction as the dividend and inverting the second fraction as the divisor.Physics 101
Physics 101AI hotaibat
╠²
This document outlines the chapters and sections covered for a Physics 101 course. It requires students to read chapters 1 through 11 in the textbook Physics for Scientists and Engineers with Modern Physics by Serway, 6th edition, excluding specific sections in each chapter, such as sections 1.2, 1.6, and 1.7 in chapter 1 on Physics and Measurement.Quantum calculations and calculational chemistry
Quantum calculations and calculational chemistrynazanin25
╠²
This document discusses computational chemistry and different methods for calculating molecular structure and properties using computers. It describes two main approaches: molecular mechanics, which views molecules as collections of atoms and calculates potential energy based on bonding parameters; and quantum mechanics, which uses the Schrodinger equation and approximations like Born-Oppenheimer and molecular orbital theory. Specific quantum methods discussed include semi-empirical, ab initio, and density functional theory. Popular computational programs and visualization software are also listed.Mole, avogadro's number and calculations based on balanced chemical equation
Mole, avogadro's number and calculations based on balanced chemical equationInternational advisers
╠²
chemistryCw stoichiometry intro 041112
Cw stoichiometry intro 041112Gandaki Boarding School,Lamachaur-16 Pokhara, Nepal
╠²
The document provides an introduction to stoichiometry and the mole concept. It discusses key topics including:
1. The mole is a unit used to describe the amount of substance in chemistry and is equal to 6.022x1023 particles.
2. The molar mass of an element or compound is the mass in grams of one mole and can be used to calculate amounts in chemical reactions.
3. Conversions can be made between moles, particles, masses, and volumes using the molar mass and molar relationships like moles = mass/molar mass.
4. Solution concentration is expressed in molarity, which is the moles of solute per liter of solution. Mchemistry Gcse Chapter 4stoichiometry/Quantitative chemistry .pptx
chemistry Gcse Chapter 4stoichiometry/Quantitative chemistry .pptxAnumToqueer
╠²
The document provides information on stoichiometry, including:
- Relative atomic mass is the average mass of atoms of an element taking into account isotopes, measured on a scale where carbon-12 is 12.
- Relative formula mass is the sum of the relative atomic masses of all the atoms in a chemical formula.
- A mole is the amount of a substance containing 6.02x10^23 particles like atoms or molecules. This allows for easy calculation of amounts in chemical reactions.
- Stoichiometry uses molar ratios from balanced chemical equations to calculate amounts of reactants and products in terms of moles and masses. The mole concept and molar ratios allow for determining reacting masses and deduChem.pptx
Chem.pptxMarjorieRoseTeodosio
╠²
Stoichiometry is the study of quantitative relationships between amounts of substances involved in chemical reactions. It allows chemists to determine mole and particle quantities. The mole is the standard unit for measuring amounts of substances and refers to 6.022x1023 elementary entities. Molar mass is the mass of one mole of a substance and is calculated differently for elements versus compounds. Percent composition by mass can be determined by dividing the mass of each element by the total molar mass. Empirical and molecular formulas relate the simplest and actual ratios of elements in a compound.Ppt 2 S1.4Counting particles by mass The mole [Autosaved].pptx
Ppt 2 S1.4Counting particles by mass The mole [Autosaved].pptxNeera16
╠²
The document discusses the concept of the mole as the SI unit for measuring amounts of substances, emphasizing its role in quantifying matter at the atomic scale using Avogadro's number (6.02 x 10^23). It outlines methods for calculating molar mass, empirical and molecular formulas, as well as techniques for determining the concentration of solutions. Additionally, the document includes practice questions and examples related to these chemical calculations.The mole concept
The mole conceptQuazanne van der Bijl
╠²
The mole is a unit used in chemistry to express amounts of substances. It represents 6.022x10^23 elementary entities, such as atoms, molecules, ions or other particles of a substance. This number is known as Avogadro's constant, after scientist Amedeo Avogadro who proposed that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. The mass of one mole of a substance, known as its molar mass, can be used to determine the number of moles in a given mass of that substance and vice versa through calculation.MOLE CONCEPT AND CONCENTRATION TERMS CLASS 11 CHEMISTRY CHAPTER-1
MOLE CONCEPT AND CONCENTRATION TERMS CLASS 11 CHEMISTRY CHAPTER-1JAYSHREE SAMANTA
╠²
- Atomic mass is defined as one-twelfth the mass of one atom of carbon-12. Molecular mass is the sum of the atomic masses of the elements in a molecule.
- One mole is equal to 6.022 x 1023 particles. The number of moles can be calculated by dividing the number of particles by Avogadro's number.
- Molarity is moles of solute per liter of solution and depends on volume. Molality is moles of solute per kg of solvent and depends on mass. Temperature affects molarity but not molality.
- The limiting reagent is the reactant present in the least amount that limits the amount of product that canTHE MOLE.pptx
THE MOLE.pptxBernadetteArao1
╠²
This document discusses the mole concept in chemistry. It defines the mole as the basic unit used to count particles like atoms and molecules, with 1 mole equaling 6.02 x 1023 particles. The mole is used to convert between numbers of particles and mass amounts in grams. It relates the mole to Avogadro's number which was introduced by Amadeo Avogadro in 1811. Molar mass refers to the mass in grams of 1 mole of a substance and is equivalent to the molecular weight. Examples are given for converting between moles, particles, and mass amounts.Chapter - 3, Atoms And Molecules, (Mole Concept) Science, Class 9
Chapter - 3, Atoms And Molecules, (Mole Concept) Science, Class 9Shivam Parmar
╠²
The document explains the concept of a mole in chemistry, which is a unit for measuring large quantities of small entities like atoms and molecules, specifically highlighting that 1 mole corresponds to 6.022 x 10^23 particles and the molecular mass of a substance in grams. With examples using sodium chloride and water, it clarifies how to calculate moles and their relationship to atomic mass and Avogadro's number. Additionally, it outlines the historical context of the term 'mole' and its significance in chemical reactions.This activity is designed to introduce a convenient unit used by.docx
This activity is designed to introduce a convenient unit used by.docxhowardh5
╠²
This document introduces the mole as a key unit for chemists, representing 6.022 x 10^23 particles, necessary for counting atoms and molecules due to their minuscule size. It explains the connection between the mass of one mole of an element and its atomic mass in grams, detailing the calculation of moles and empirical formulas through practical experiments. Additionally, it guides through the process of determining the empirical formula of magnesium oxide through a laboratory activity involving mass measurements and chemical reactions.c4e237864d12cdb0be66e0e86247e4cben4.pptx
c4e237864d12cdb0be66e0e86247e4cben4.pptxmanojkumartakshila
╠²
The document explains the mole concept, defining a mole as the amount of substance containing 6.02 x 10^23 entities, known as Avogadro's number. It discusses how to calculate molar masses, empirical formulas, and performs various mole calculations involving atomic mass and percentage composition. Additionally, it covers stoichiometry and limiting reagents in chemical reactions.Chapter 1-mole concept.ppt
Chapter 1-mole concept.pptabid masood
╠²
The document discusses the mole concept in chemistry. Some key points:
- A mole is the amount of substance containing Avogadro's number (6.022x1023) of elementary entities like atoms, molecules, formula units.
- One mole of any substance has a mass in grams equal to its formula/molar mass. For example, 1 mole of iron (Fe) has a mass of 55.85 g.
- The mole can be used to convert between the number of particles/formula units and the mass of a substance using molar mass and the definition of 1 mole.
- Common calculations include determining moles from mass or vice versa using molar mass, as well asCHEMISTRY CHAPTER 1.2 Mole Concepts.pptx
CHEMISTRY CHAPTER 1.2 Mole Concepts.pptxLokiIkol2
╠²
The document discusses various mole concepts including:
- Defining the mole and Avogadro's constant.
- Relating moles, mass, and particles for elements, compounds, and ions.
- Calculating molar mass and mass from moles.
- Relating the volume of gases to moles using Avogadro's law and molar volume.
- Explaining empirical and molecular formulas and calculating them from composition data.
- Defining different concentration units - molarity, molality, mole fraction, percentage by mass and volume.Chapter 10 - Chemical Quantities
Chapter 10 - Chemical QuantitiesGalen West
╠²
- The mole is a unit used to measure very large numbers of small particles like atoms or molecules. One mole equals 6.02 x 10^23 particles.
- The mass of one mole of an element is called its gram atomic mass (gam). The mass of one mole of a compound is called its gram molecular mass (gmm) or gram formula mass (gfm).
- At standard temperature and pressure, one mole of any gas occupies a volume of 22.4 L. This volume is known as the molar volume.Chemistry - Chp 10 - Chemical Quantities - PowerPoint
Chemistry - Chp 10 - Chemical Quantities - PowerPointMr. Walajtys
╠²
This chapter discusses the mole as a unit for measuring amounts of substances. It defines key terms like the mole, Avogadro's number, molar mass, and representative particles. It explains how to use molar mass to convert between mass and moles of a substance. The chapter also covers calculations involving chemical formulas, percent composition, and determining empirical and molecular formulas from experimental data.Ch 11 notes complete
Ch 11 notes completeEsther Herrera
╠²
The document discusses moles, molar mass, and empirical and molecular formulas.
It defines key terms like mole, Avogadro's number, and molar mass. A mole represents 6.02x1023 particles of a substance. Molar mass is the mass in grams of one mole of a substance.
Examples are provided for calculating moles from mass and vice versa using molar mass. Empirical formulas give the lowest whole number ratio of elements in a compound, while molecular formulas specify the actual number of each atom in a molecule or formula unit.Ch 11 notes complete
Ch 11 notes completeEsther Herrera
╠²
The document discusses moles, molar mass, and empirical and molecular formulas.
It defines key terms like mole, Avogadro's number, and molar mass. A mole represents 6.02x1023 particles of a substance. Molar mass is the mass in grams of one mole of a substance.
Examples are provided for calculating moles from mass and vice versa using molar mass. Empirical formulas represent the lowest whole number ratio of elements in a compound, while molecular formulas specify the actual number of each atom in a molecule or formula unit.Chemical Measurement (1).ppt
Chemical Measurement (1).pptHonneyFaithCastanare
╠²
This document discusses the mole concept in chemistry. It defines atomic mass, formula mass, and molar mass. Atomic mass is a relative scale that compares atom masses to carbon-12. Formula mass is the sum of all atom masses in a compound. Molar mass is the mass in grams of one mole of a substance. A mole is defined as Avogadro's number (6.02x1023) of particles, which allows chemists to convert between number of particles and mass.Chemistry Chapter 3
Chemistry Chapter 3tanzmanj
╠²
The document provides information about atoms and their structure. It defines key terms like protons, neutrons, electrons, nucleus and isotopes. It explains that the number of protons determines the element and distinguishes one atom from another. The mole is also defined as 6.02x10^23 particles and is used to measure amounts of substances on a macroscopic scale. Formulas are given to calculate molar mass and empirical formulas.3,stoichiometry
3,stoichiometryž╣┘ä┘Ŗ ž╣┘ä┘Ŗ
╠²
This document discusses stoichiometry, which is the quantitative study of chemical reactions. It defines key terms like atomic mass, isotopes, moles, molar mass, and percent composition. It provides examples of calculating these values, such as determining the number of atoms or moles of a substance based on its mass. The document establishes the relationships between atoms, moles, mass, and molar mass that are fundamental to stoichiometric calculations.Aprendendo Arquitetura Framework Salesforce - Dia 02
Aprendendo Arquitetura Framework Salesforce - Dia 02Mauricio Alexandre Silva
╠²
Aprendendo Arquitetura Framework Salesforce - Dia 02
List View Components in Odoo 18 - Odoo ║▌║▌▀Żs
List View Components in Odoo 18 - Odoo ║▌║▌▀ŻsCeline George
╠²
In Odoo, there are many types of views possible like List view, Kanban view, Calendar view, Pivot view, Search view, etc.
The major change that introduced in the Odoo 18 technical part in creating views is the tag <tree> got replaced with the <list> for creating list views. More Related Content
Similar to Ib quan chemistry(mole concept) (20)
Cw stoichiometry intro 041112
Cw stoichiometry intro 041112Gandaki Boarding School,Lamachaur-16 Pokhara, Nepal
╠²
The document provides an introduction to stoichiometry and the mole concept. It discusses key topics including:
1. The mole is a unit used to describe the amount of substance in chemistry and is equal to 6.022x1023 particles.
2. The molar mass of an element or compound is the mass in grams of one mole and can be used to calculate amounts in chemical reactions.
3. Conversions can be made between moles, particles, masses, and volumes using the molar mass and molar relationships like moles = mass/molar mass.
4. Solution concentration is expressed in molarity, which is the moles of solute per liter of solution. Mchemistry Gcse Chapter 4stoichiometry/Quantitative chemistry .pptx
chemistry Gcse Chapter 4stoichiometry/Quantitative chemistry .pptxAnumToqueer
╠²
The document provides information on stoichiometry, including:
- Relative atomic mass is the average mass of atoms of an element taking into account isotopes, measured on a scale where carbon-12 is 12.
- Relative formula mass is the sum of the relative atomic masses of all the atoms in a chemical formula.
- A mole is the amount of a substance containing 6.02x10^23 particles like atoms or molecules. This allows for easy calculation of amounts in chemical reactions.
- Stoichiometry uses molar ratios from balanced chemical equations to calculate amounts of reactants and products in terms of moles and masses. The mole concept and molar ratios allow for determining reacting masses and deduChem.pptx
Chem.pptxMarjorieRoseTeodosio
╠²
Stoichiometry is the study of quantitative relationships between amounts of substances involved in chemical reactions. It allows chemists to determine mole and particle quantities. The mole is the standard unit for measuring amounts of substances and refers to 6.022x1023 elementary entities. Molar mass is the mass of one mole of a substance and is calculated differently for elements versus compounds. Percent composition by mass can be determined by dividing the mass of each element by the total molar mass. Empirical and molecular formulas relate the simplest and actual ratios of elements in a compound.Ppt 2 S1.4Counting particles by mass The mole [Autosaved].pptx
Ppt 2 S1.4Counting particles by mass The mole [Autosaved].pptxNeera16
╠²
The document discusses the concept of the mole as the SI unit for measuring amounts of substances, emphasizing its role in quantifying matter at the atomic scale using Avogadro's number (6.02 x 10^23). It outlines methods for calculating molar mass, empirical and molecular formulas, as well as techniques for determining the concentration of solutions. Additionally, the document includes practice questions and examples related to these chemical calculations.The mole concept
The mole conceptQuazanne van der Bijl
╠²
The mole is a unit used in chemistry to express amounts of substances. It represents 6.022x10^23 elementary entities, such as atoms, molecules, ions or other particles of a substance. This number is known as Avogadro's constant, after scientist Amedeo Avogadro who proposed that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. The mass of one mole of a substance, known as its molar mass, can be used to determine the number of moles in a given mass of that substance and vice versa through calculation.MOLE CONCEPT AND CONCENTRATION TERMS CLASS 11 CHEMISTRY CHAPTER-1
MOLE CONCEPT AND CONCENTRATION TERMS CLASS 11 CHEMISTRY CHAPTER-1JAYSHREE SAMANTA
╠²
- Atomic mass is defined as one-twelfth the mass of one atom of carbon-12. Molecular mass is the sum of the atomic masses of the elements in a molecule.
- One mole is equal to 6.022 x 1023 particles. The number of moles can be calculated by dividing the number of particles by Avogadro's number.
- Molarity is moles of solute per liter of solution and depends on volume. Molality is moles of solute per kg of solvent and depends on mass. Temperature affects molarity but not molality.
- The limiting reagent is the reactant present in the least amount that limits the amount of product that canTHE MOLE.pptx
THE MOLE.pptxBernadetteArao1
╠²
This document discusses the mole concept in chemistry. It defines the mole as the basic unit used to count particles like atoms and molecules, with 1 mole equaling 6.02 x 1023 particles. The mole is used to convert between numbers of particles and mass amounts in grams. It relates the mole to Avogadro's number which was introduced by Amadeo Avogadro in 1811. Molar mass refers to the mass in grams of 1 mole of a substance and is equivalent to the molecular weight. Examples are given for converting between moles, particles, and mass amounts.Chapter - 3, Atoms And Molecules, (Mole Concept) Science, Class 9
Chapter - 3, Atoms And Molecules, (Mole Concept) Science, Class 9Shivam Parmar
╠²
The document explains the concept of a mole in chemistry, which is a unit for measuring large quantities of small entities like atoms and molecules, specifically highlighting that 1 mole corresponds to 6.022 x 10^23 particles and the molecular mass of a substance in grams. With examples using sodium chloride and water, it clarifies how to calculate moles and their relationship to atomic mass and Avogadro's number. Additionally, it outlines the historical context of the term 'mole' and its significance in chemical reactions.This activity is designed to introduce a convenient unit used by.docx
This activity is designed to introduce a convenient unit used by.docxhowardh5
╠²
This document introduces the mole as a key unit for chemists, representing 6.022 x 10^23 particles, necessary for counting atoms and molecules due to their minuscule size. It explains the connection between the mass of one mole of an element and its atomic mass in grams, detailing the calculation of moles and empirical formulas through practical experiments. Additionally, it guides through the process of determining the empirical formula of magnesium oxide through a laboratory activity involving mass measurements and chemical reactions.c4e237864d12cdb0be66e0e86247e4cben4.pptx
c4e237864d12cdb0be66e0e86247e4cben4.pptxmanojkumartakshila
╠²
The document explains the mole concept, defining a mole as the amount of substance containing 6.02 x 10^23 entities, known as Avogadro's number. It discusses how to calculate molar masses, empirical formulas, and performs various mole calculations involving atomic mass and percentage composition. Additionally, it covers stoichiometry and limiting reagents in chemical reactions.Chapter 1-mole concept.ppt
Chapter 1-mole concept.pptabid masood
╠²
The document discusses the mole concept in chemistry. Some key points:
- A mole is the amount of substance containing Avogadro's number (6.022x1023) of elementary entities like atoms, molecules, formula units.
- One mole of any substance has a mass in grams equal to its formula/molar mass. For example, 1 mole of iron (Fe) has a mass of 55.85 g.
- The mole can be used to convert between the number of particles/formula units and the mass of a substance using molar mass and the definition of 1 mole.
- Common calculations include determining moles from mass or vice versa using molar mass, as well asCHEMISTRY CHAPTER 1.2 Mole Concepts.pptx
CHEMISTRY CHAPTER 1.2 Mole Concepts.pptxLokiIkol2
╠²
The document discusses various mole concepts including:
- Defining the mole and Avogadro's constant.
- Relating moles, mass, and particles for elements, compounds, and ions.
- Calculating molar mass and mass from moles.
- Relating the volume of gases to moles using Avogadro's law and molar volume.
- Explaining empirical and molecular formulas and calculating them from composition data.
- Defining different concentration units - molarity, molality, mole fraction, percentage by mass and volume.Chapter 10 - Chemical Quantities
Chapter 10 - Chemical QuantitiesGalen West
╠²
- The mole is a unit used to measure very large numbers of small particles like atoms or molecules. One mole equals 6.02 x 10^23 particles.
- The mass of one mole of an element is called its gram atomic mass (gam). The mass of one mole of a compound is called its gram molecular mass (gmm) or gram formula mass (gfm).
- At standard temperature and pressure, one mole of any gas occupies a volume of 22.4 L. This volume is known as the molar volume.Chemistry - Chp 10 - Chemical Quantities - PowerPoint
Chemistry - Chp 10 - Chemical Quantities - PowerPointMr. Walajtys
╠²
This chapter discusses the mole as a unit for measuring amounts of substances. It defines key terms like the mole, Avogadro's number, molar mass, and representative particles. It explains how to use molar mass to convert between mass and moles of a substance. The chapter also covers calculations involving chemical formulas, percent composition, and determining empirical and molecular formulas from experimental data.Ch 11 notes complete
Ch 11 notes completeEsther Herrera
╠²
The document discusses moles, molar mass, and empirical and molecular formulas.
It defines key terms like mole, Avogadro's number, and molar mass. A mole represents 6.02x1023 particles of a substance. Molar mass is the mass in grams of one mole of a substance.
Examples are provided for calculating moles from mass and vice versa using molar mass. Empirical formulas give the lowest whole number ratio of elements in a compound, while molecular formulas specify the actual number of each atom in a molecule or formula unit.Ch 11 notes complete
Ch 11 notes completeEsther Herrera
╠²
The document discusses moles, molar mass, and empirical and molecular formulas.
It defines key terms like mole, Avogadro's number, and molar mass. A mole represents 6.02x1023 particles of a substance. Molar mass is the mass in grams of one mole of a substance.
Examples are provided for calculating moles from mass and vice versa using molar mass. Empirical formulas represent the lowest whole number ratio of elements in a compound, while molecular formulas specify the actual number of each atom in a molecule or formula unit.Chemical Measurement (1).ppt
Chemical Measurement (1).pptHonneyFaithCastanare
╠²
This document discusses the mole concept in chemistry. It defines atomic mass, formula mass, and molar mass. Atomic mass is a relative scale that compares atom masses to carbon-12. Formula mass is the sum of all atom masses in a compound. Molar mass is the mass in grams of one mole of a substance. A mole is defined as Avogadro's number (6.02x1023) of particles, which allows chemists to convert between number of particles and mass.Chemistry Chapter 3
Chemistry Chapter 3tanzmanj
╠²
The document provides information about atoms and their structure. It defines key terms like protons, neutrons, electrons, nucleus and isotopes. It explains that the number of protons determines the element and distinguishes one atom from another. The mole is also defined as 6.02x10^23 particles and is used to measure amounts of substances on a macroscopic scale. Formulas are given to calculate molar mass and empirical formulas.3,stoichiometry
3,stoichiometryž╣┘ä┘Ŗ ž╣┘ä┘Ŗ
╠²
This document discusses stoichiometry, which is the quantitative study of chemical reactions. It defines key terms like atomic mass, isotopes, moles, molar mass, and percent composition. It provides examples of calculating these values, such as determining the number of atoms or moles of a substance based on its mass. The document establishes the relationships between atoms, moles, mass, and molar mass that are fundamental to stoichiometric calculations.Recently uploaded (20)
Aprendendo Arquitetura Framework Salesforce - Dia 02
Aprendendo Arquitetura Framework Salesforce - Dia 02Mauricio Alexandre Silva
╠²
Aprendendo Arquitetura Framework Salesforce - Dia 02
List View Components in Odoo 18 - Odoo ║▌║▌▀Żs
List View Components in Odoo 18 - Odoo ║▌║▌▀ŻsCeline George
╠²
In Odoo, there are many types of views possible like List view, Kanban view, Calendar view, Pivot view, Search view, etc.
The major change that introduced in the Odoo 18 technical part in creating views is the tag <tree> got replaced with the <list> for creating list views. Code Profiling in Odoo 18 - Odoo 18 ║▌║▌▀Żs
Code Profiling in Odoo 18 - Odoo 18 ║▌║▌▀ŻsCeline George
╠²
Profiling in Odoo identifies slow code and resource-heavy processes, ensuring better system performance. Odoo code profiling detects bottlenecks in custom modules, making it easier to improve speed and scalability.University of Ghana Cracks Down on Misconduct: Over 100 Students Sanctioned
University of Ghana Cracks Down on Misconduct: Over 100 Students SanctionedKweku Zurek
╠²
University of Ghana Cracks Down on Misconduct: Over 100 Students Sanctioned
INDUCTIVE EFFECT slide for first prof pharamacy students
INDUCTIVE EFFECT slide for first prof pharamacy studentsSHABNAM FAIZ
╠²
The inductive effect is the electron-withdrawing or electron-donating effect transmitted through sigma (Žā) bonds in a molecule due to differences in electronegativity between atoms.
---
¤ö╣ Definition:
The inductive effect is the permanent shifting of electrons in a sigma bond caused by the electronegativity difference of atoms, resulting in partial charges within the molecule.How to Customize Quotation Layouts in Odoo 18
How to Customize Quotation Layouts in Odoo 18Celine George
╠²
Customizing quotation layouts in Odoo 18 allows businesses to personalize their quotations to match branding or specific requirements. This can include adding logos, custom fields, or modifying headers and footers. Paper 107 | From Watchdog to Lapdog: IshiguroŌĆÖs Fiction and the Rise of ŌĆ£Godi...
Paper 107 | From Watchdog to Lapdog: IshiguroŌĆÖs Fiction and the Rise of ŌĆ£Godi...Rajdeep Bavaliya
╠²
Dive into a captivating analysis where Kazuo IshiguroŌĆÖs nuanced fiction meets the stark realities of postŌĆæ2014 Indian journalism. Uncover how ŌĆ£Godi MediaŌĆØ turned from watchdog to lapdog, echoing the moral compromises of IshiguroŌĆÖs protagonists. WeŌĆÖll draw parallels between restrained narrative silences and sensationalist headlinesŌĆöare our media heroes or traitors? DonŌĆÖt forget to follow for more deep dives!
M.A. Sem - 2 | Presentation
Presentation Season - 2
Paper - 107: The Twentieth Century Literature: From World War II to the End of the Century
Submitted Date: April 4, 2025
Paper Name: The Twentieth Century Literature: From World War II to the End of the Century
Topic: From Watchdog to Lapdog: IshiguroŌĆÖs Fiction and the Rise of ŌĆ£Godi MediaŌĆØ in Post-2014 Indian Journalism
[Please copy the link and paste it into any web browser to access the content.]
Video Link: https://youtu.be/kIEqwzhHJ54
For a more in-depth discussion of this presentation, please visit the full blog post at the following link: https://rajdeepbavaliya2.blogspot.com/2025/04/from-watchdog-to-lapdog-ishiguro-s-fiction-and-the-rise-of-godi-media-in-post-2014-indian-journalism.html
Please visit this blog to explore additional presentations from this season:
Hashtags:
#GodiMedia #Ishiguro #MediaEthics #WatchdogVsLapdog #IndianJournalism #PressFreedom #LiteraryCritique #AnArtistOfTheFloatingWorld #MediaCapture #KazuoIshiguro
Keyword Tags:
Godi Media, Ishiguro fiction, post-2014 Indian journalism, media capture, Kazuo Ishiguro analysis, watchdog to lapdog, press freedom India, media ethics, literature and media, An Artist of the Floating WorldHow payment terms are configured in Odoo 18
How payment terms are configured in Odoo 18Celine George
╠²
Payment terms in Odoo 18 help define the conditions for when invoices are due. This feature can split payments into multiple parts and automate due dates based on specific rules.K12 Tableau User Group virtual event June 18, 2025
K12 Tableau User Group virtual event June 18, 2025dogden2
╠²
National K12 Tableau User Group: June 2025 meeting slidesYSPH VMOC Special Report - Measles Outbreak Southwest US 6-14-2025.pptx
YSPH VMOC Special Report - Measles Outbreak Southwest US 6-14-2025.pptxYale School of Public Health - The Virtual Medical Operations Center (VMOC)
╠²
BLUF:
The Texas outbreak has slowed down, but sporadic cases continue to emerge in Kansas, Oklahoma, and New Mexico.
Elsewhere in the US, we continue to see signs of acceleration due to outbreaks outside the Southwest (North Dakota, Montana, and Colorado) and travel-related cases. Measles exposures due to travel are expected to pose a significant challenge throughout the summer.
The U.S. is on track to exceed its 30-year high for measles cases (1,274) within the next two weeks.
Here is the latest update:
CURRENT CASE COUNT: 919
ŌĆóTexas: 744 (+2) (55% of cases are in Gaines County).
ŌĆóNew Mexico: 81 (83% of cases are from Lea County).
ŌĆóOklahoma: 20 (+2)
ŌĆóKansas: 74 (+5) (38.89% of the cases are from Gray County).
HOSPITALIZATIONS: 104
ŌĆó Texas: 96 (+2) ŌĆō This accounts for 13% of all cases in Texas.
ŌĆó New Mexico: 7 ŌĆō This accounts for 9.47% of all cases in New Mexico.
ŌĆó Kansas: 3 ŌĆō This accounts for 5.08% of all cases in the state of Kansas.
DEATHS: 3
ŌĆóTexas: 2 ŌĆō This is 0.27% of all cases in Texas.
ŌĆóNew Mexico: 1 ŌĆō This is 1.23% of all cases in New Mexico.
US NATIONAL CASE COUNT: 1,197
INTERNATIONAL SPREAD
ŌĆóMexico: 2337 (+257), 5 fatalities
ŌĆÆChihuahua, Mexico: 2,179 (+239) cases, 4 fatalities, 7 currently hospitalized.
ŌĆóCanada: 3,207 (+208), 1 fatality
ŌĆÆOntario Outbreak, Canada: 2,115 (+74) cases, 158 hospitalizations, 1 fatality.
ŌĆÆAlberta, Canada: 879(+118) cases, 5 currently hospitalized.How to use search fetch method in Odoo 18
How to use search fetch method in Odoo 18Celine George
╠²
The search_fetch is a powerful ORM method used in Odoo for some specific addons to combine the functionality of search and read for more efficient data fetching. It might be used to search for records and fetch specific fields in a single call. It stores the result in the cache memory.Great Governors' Send-Off Quiz 2025 Prelims IIT KGP
Great Governors' Send-Off Quiz 2025 Prelims IIT KGPIIT Kharagpur Quiz Club
╠²
Prelims of the Great Governors' Send-Off Quiz 2025 hosted by the outgoing governors.
QMs: Aarushi, Aatir, Aditya, ArnavF-BLOCK ELEMENTS POWER POINT PRESENTATIONS
F-BLOCK ELEMENTS POWER POINT PRESENTATIONSmprpgcwa2024
╠²
F-block elements are a group of elements in the periodic table that have partially filled f-orbitals. They are also known as inner transition elements. F-block elements are divided into two series:
1.Lanthanides (La- Lu) These elements are also known as rare earth elements.
2.Actinides (Ac- Lr): These elements are radioactive and have complex electronic configurations.
F-block elements exhibit multiple oxidation states due to the availability of f-orbitals.
2. Many f-block compounds are colored due to f-f transitions.
3. F-block elements often exhibit paramagnetic or ferromagnetic behavior.4. Actinides are radioactive.
F-block elements are used as catalysts in various industrial processes.
Actinides are used in nuclear reactors and nuclear medicine.
F-block elements are used in lasers and phosphors due to their luminescent properties.
F-block elements have unique electronic and magnetic properties.Romanticism in Love and Sacrifice An Analysis of Oscar WildeŌĆÖs The Nightingal...
Romanticism in Love and Sacrifice An Analysis of Oscar WildeŌĆÖs The Nightingal...KaryanaTantri21
╠²
The story revolves around a college student who despairs not having a red rose as a condition for dancing with the girl he loves. The nightingale hears his complaint and offers to create the red rose at the cost of his life. He sang a love song all night with his chest stuck to the thorns of the rose tree. Finally, the red rose grew, but his sacrifice was in vain. The girl rejected the flower because it didnŌĆÖt match her outfit and preferred a jewellery gift. The student threw the flower on the street and returned to studying philosophyIntellectual Property Right (Jurisprudence).pptx
Intellectual Property Right (Jurisprudence).pptxVishal Chanalia
╠²
Intellectual property corresponds to ideas owned by a person or a firm and thus subjected to legal protection under the law.
The main purpose of intellectual property is to give encouragement to the innovators of new concepts by giving them the opportunity to make sufficient profits from their inventions and recover their manufacturing costs and efforts. June 2025 Progress Update With Board Call_In process.pptx
June 2025 Progress Update With Board Call_In process.pptxInternational Society of Service Innovation Professionals
╠²
---
June 25 ISSIP Event - slides in process
20250618 PPre-Event Presentation Summary - Progress Update with Board Series June 25
ISSIP Website Upcoming Events Description: https://issip.org/event/semi-annual-issip-progress-call/
Register here (even if you cannot attend live online, all who register will get link to recording and slides post-event): https://docs.google.com/forms/d/e/1FAIpQLSdThrop1rafOCo4PQkYiS2XApclJuMjYONEHRMGBsceRdcQqg/viewform
This pre-event presentation: /slideshow/june-2025-progress-update-with-board-call_in-process-pptx/280718770
This pre-event recording: https://youtu.be/Shjgd5o488o
---Paper 107 | From Watchdog to Lapdog: IshiguroŌĆÖs Fiction and the Rise of ŌĆ£Godi...
Paper 107 | From Watchdog to Lapdog: IshiguroŌĆÖs Fiction and the Rise of ŌĆ£Godi...Rajdeep Bavaliya
╠²
YSPH VMOC Special Report - Measles Outbreak Southwest US 6-14-2025.pptx
YSPH VMOC Special Report - Measles Outbreak Southwest US 6-14-2025.pptxYale School of Public Health - The Virtual Medical Operations Center (VMOC)
╠²
June 2025 Progress Update With Board Call_In process.pptx
June 2025 Progress Update With Board Call_In process.pptxInternational Society of Service Innovation Professionals
╠²
Ad
Ib quan chemistry(mole concept)
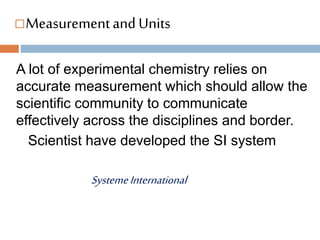
- 2. ’é©Measurementand Units A lot of experimental chemistry relies on accurate measurement which should allow the scientific community to communicate effectively across the disciplines and border. Scientist have developed the SI system SystemeInternational
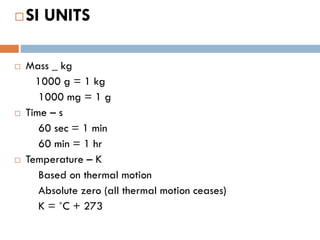
- 3. ’é©SI UNITS ’é© Mass _ kg 1000 g = 1 kg 1000 mg = 1 g ’é© Time ŌĆō s 60 sec = 1 min 60 min = 1 hr ’é© Temperature ŌĆō K Based on thermal motion Absolute zero (all thermal motion ceases) K = ╦ÜC + 273
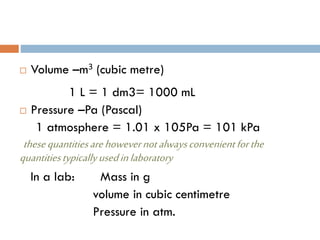
- 4. ’é© Volume ŌĆōm3 (cubic metre) 1 L = 1 dm3= 1000 mL ’é© Pressure ŌĆōPa (Pascal) 1 atmosphere = 1.01 x 105Pa = 101 kPa thesequantitiesarehowevernotalwaysconvenientforthe quantitiestypicallyusedinlaboratory In a lab: Mass in g volume in cubic centimetre Pressure in atm.
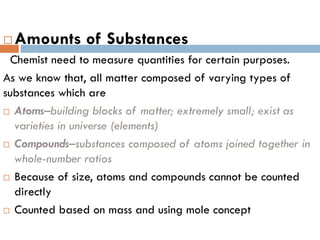
- 5. ’é© Amounts of Substances Chemist need to measure quantities for certain purposes. As we know that, all matter composed of varying types of substances which are ’é© AtomsŌĆōbuilding blocks of matter; extremely small; exist as varieties in universe (elements) ’é© CompoundsŌĆōsubstances composed of atoms joined together in whole-number ratios ’é© Because of size, atoms and compounds cannot be counted directly ’é© Counted based on mass and using mole concept
- 6. THE MOLE WHAT IS A MOLE ? it is the standard unit of amount of a substance - it is just a number, a very big number it is a way of saying a number in words, just like... DOZEN for 12 SCORE for 20 GROSS for 144 HOW BIG IS IT ? 602200000000000000000000 (Approximately)... THATŌĆÖS BIG !!! It is a lot easier to write it as... 6.022 x 1023 ’é© It doesnŌĆÖt matter what the number is as long as everybody sticks to the same value
- 7. Avogadro No. or MOLE ’é© The ratios with which elements combine depend on the number of atoms not on their mass. ’é© Individual atoms and molecules are extremely small. Hence a larger unit is appropriate for measuring quantities of matter. ’é© A mole is the amount of substance which contains the same no. of chemical sps as there are atoms in exactly 12 g of the C-12 . Its S.I. unit is mol. This number is known as AvogadroŌĆÖs constant. 1 mole is equal to 6.02 x 1023 particles. One gram of H atoms contains 6.02 x 1023 atoms.
- 8. THE MOLE WHY USE IT ? Atoms and molecules donŌĆÖt weigh much so it is easier to count large numbers of them. In fact it is easier to weigh substances. Using moles tells you... how many particles you get in a certain mass the mass of a certain number of particles DO I NEED TO KNOW ANYTHING ELSE ? Yes, it would help if you can balance equations AND Keep trying, you will get the idea ... EVENTUALLY!
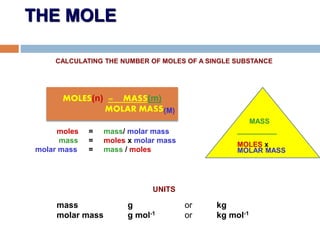
- 9. THE MOLE CALCULATING THE NUMBER OF MOLES OF A SINGLE SUBSTANCE moles = mass/ molar mass mass = moles x molar mass molar mass = mass / moles UNITS mass g or kg molar mass g mol-1 or kg mol-1 MOLES(n) = MASS(m) MOLAR MASS MASS __________ MOLES x MOLAR MASS
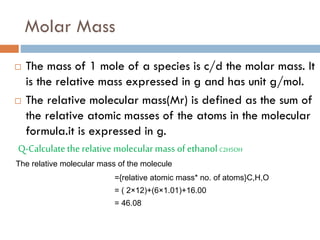
- 10. Molar Mass ’é© The mass of 1 mole of a species is c/d the molar mass. It is the relative mass expressed in g and has unit g/mol. ’é© The relative molecular mass(Mr) is defined as the sum of the relative atomic masses of the atoms in the molecular formula.it is expressed in g. Q-Calculatethe relative molecular mass ofethanol C2H5OH The relative molecular mass of the molecule ={relative atomic mass* no. of atoms}C,H,O = ( 2├Ś12)+(6├Ś1.01)+16.00 = 46.08
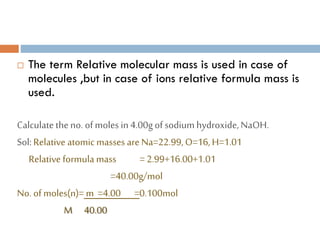
- 11. ’é© The term Relative molecular mass is used in case of molecules ,but in case of ions relative formula mass is used. Calculatetheno. ofmoles in4.00gof sodium hydroxide,NaOH. Sol:Relative atomic masses are Na=22.99,O=16,H=1.01 Relative formula mass =2.99+16.00+1.01 =40.00g/mol No. of moles(n)= m =4.00 =0.100mol M 40.00
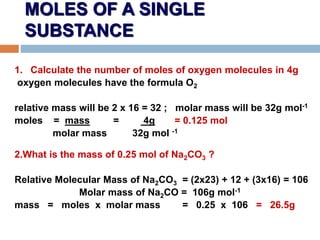
- 12. MOLES OF A SINGLE SUBSTANCE 1. Calculate the number of moles of oxygen molecules in 4g oxygen molecules have the formula O2 relative mass will be 2 x 16 = 32 ; molar mass will be 32g mol-1 moles = mass = 4g = 0.125 mol molar mass 32g mol -1 2.What is the mass of 0.25 mol of Na2CO3 ? Relative Molecular Mass of Na2CO3 = (2x23) + 12 + (3x16) = 106 Molar mass of Na2CO = 106g mol-1 mass = moles x molar mass = 0.25 x 106 = 26.5g
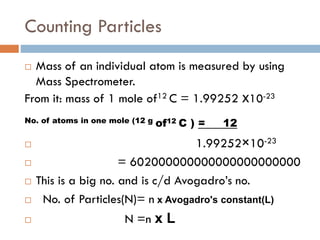
- 13. Counting Particles ’é© Mass of an individual atom is measured by using Mass Spectrometer. From it: mass of 1 mole of12 C = 1.99252 x10-23 No. of atoms in one mole (12 g of12 C ) = 12 ’é© 1.99252├Ś10-23 ’é© = 602000000000000000000000 ’é© This is a big no. and is c/d AvogadroŌĆÖs no. ’é© No. of Particles(N)= n x Avogadro's constant(L) ’é© N =n x L
- 14. ’é© Calculate the amount of water,H2O that contains 1.80 x 1024. ’é© N=n x L ’é© = 1.80 x 1024 =2.99 mol 6.02x1023 (the answer should be given to 3 significant figure- the same precision as the data given in the question. If the amount given was1.8x 1024 the correct answer would be 3.0
- 15. ’é© Calculate how many hydrogen atoms are present in 3.0 moles of ethanol( C2H5OH). ’é© n= In 1 molecule of ethanol there are 6 H atoms ’é© In 1 mole of ethanol ther are 6 moles of H atoms ’é© In 3 moles of ethanol there are 6├Ś3 =18 moles of H atoms ’é© N = nL ’é© N =18 ├Ś6.023├Ś1023 ’é© = 1.08├Ś1025
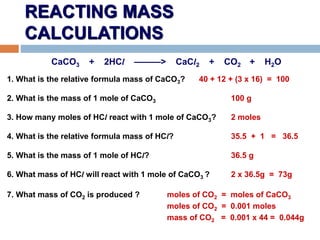
- 16. REACTING MASS CALCULATIONS CaCO3 + 2HCl ŌĆöŌĆöŌĆö> CaCl2 + CO2 + H2O 1. What is the relative formula mass of CaCO3? 40 + 12 + (3 x 16) = 100 2. What is the mass of 1 mole of CaCO3 100 g 3. How many moles of HCl react with 1 mole of CaCO3? 2 moles 4. What is the relative formula mass of HCl? 35.5 + 1 = 36.5 5. What is the mass of 1 mole of HCl? 36.5 g 6. What mass of HCl will react with 1 mole of CaCO3 ? 2 x 36.5g = 73g 7. What mass of CO2 is produced ? moles of CO2 = moles of CaCO3 moles of CO2 = 0.001 moles mass of CO2 = 0.001 x 44 = 0.044g
