Kimia analisis kelompok 4 kelas 11.5
1 like516 views
Iodimetry is a titration technique used to determine the amount of a reductant (substance being titrated) using a standardized iodine solution as the oxidizing titrant. The reductant is oxidized by iodine in a redox reaction. Iodimetry is performed under neutral or weak acid conditions to avoid the disproportionation or oxidation of iodine. Common indicators used include starch solution or carbon tetrachloride.
1 of 14












Ad
Recommended
Iodine titration by krishna baokar
Iodine titration by krishna baokarkrishnabaokar
Ėý
This document provides information on iodine titrations, including how they are used for both direct (iodimetry) and indirect (iodometry) titrations. It discusses the solubility of iodine, conditions and precautions for iodine titrations, various methods for determining the endpoint, and applications like assaying ascorbic acid, potassium permanganate, and other compounds. The document also describes how iodine solutions are standardized, often using sodium thiosulfate, as well as how sodium thiosulfate itself must be standardized due to its instability.Iodimetry & iodometry
Iodimetry & iodometryRenjithaJR1
Ėý
This document discusses iodine titrations, including iodimetric and iodometric titrations. Iodimetric titrations use iodine as the oxidizing agent, which reacts with strong reductants. Iodometric titrations involve liberating iodine from potassium iodide using an acid, then titrating the free iodine with sodium thiosulfate. Starch is commonly used as an indicator, turning blue when iodine is present. The document also provides procedures for preparing standard iodine and sodium thiosulfate solutions and describes how to standardize them using arsenic trioxide or potassium iodate, respectively.Iodimetry & iodometry
Iodimetry & iodometryApusi Chowdhury
Ėý
Iodometry and iodimetry are titration methods that involve the reaction of iodine with an analyte. In iodometry, the analyte is an oxidizing agent that liberates iodine, which is then titrated with a standard thiosulfate solution. In iodimetry, the analyte is a reducing agent that is directly titrated with a standard iodine solution. Starch is commonly used as an indicator for the titrations. Iodometric and iodimetric titrations cannot be performed in strong acidic or basic conditions due to side reactions involving iodine and interference with the indicators. The methods are useful for determining concentrations of substances like vitamin C,Iodometry & Iodimetry
Iodometry & IodimetryJoyshankar Baidya
Ėý
Iodometry and iodimetry are indirect titration methods that use iodine. In iodometry, an oxidizing agent reacts with excess potassium iodide to liberate iodine, which is then titrated against a standard reducing agent like thiosulphate. Iodimetry directly titrates a reducing analyte against a standard iodine solution. Starch indicator turns blue or violet in the presence of iodine but is colorless when all iodine reacts at the endpoint of the titration. These methods are used to determine the concentration of various chemicals like vitamin C and thiosulphate.Chemical Properties of Nitric Acid
Chemical Properties of Nitric AcidSidra Javed
Ėý
HNO3 has three main chemical properties: acidic, nitrating, and oxidizing. As an acid, it donates one H+ ion and neutralizes bases. Its nitrating property allows it to add nitro groups to organic compounds like benzene. As a strong oxidizer, HNO3's nitrogen changes oxidation states and can oxidize metals and nonmetals, reducing itself to NO2, NO or N2O depending on conditions like concentration and reactivity of the substance. Its importance lies in uses like fertilizers, explosives, and dynamite where its acidity, nitrating, or oxidizing abilities are exploited.Hydrates lecture
Hydrates lectureLeah Bensimon
Ėý
This document discusses hydrates, which are compounds that have water molecules bound to their atoms. Hydrates can be named using the compound name followed by a dot and the number of water molecules. The water molecules can be removed through heating, changing the hydrate into its anhydrous form. To determine the formula of a hydrate, the moles of water lost upon heating are calculated and compared to the moles of the compound to obtain a molar ratio, which is used to derive the chemical formula. Examples are provided to demonstrate solving for the formula and name of hydrates. Finally, some common uses of hydrates as drying agents and in solar energy are mentioned.HYDRIDES OF NITROGEN
HYDRIDES OF NITROGENSwati Singh
Ėý
This document discusses hydrides of nitrogen, including ammonia and hydrazine. It provides details on their structures, properties, preparation methods, and uses. Ammonia is a colorless gas that is lighter than air. It is prepared through reactions involving ammonium chloride or metal nitrides. Ammonia is used as a fertilizer and cleaning product. Hydrazine is a reducing agent that exists as a colorless liquid. It is prepared through reactions of sodium hypochlorite with ammonia or urea. Both compounds are discussed in terms of their molecular structures and chemical properties.Hydrogen
HydrogenRehan Daniel
Ėý
This document discusses isotopes of hydrogen, including their properties and natural occurrences. It specifically covers the three main isotopes:
1) Protium (1H1) is the most common isotope of hydrogen, making up 99.98% of naturally occurring hydrogen. It has one proton and no neutrons.
2) Deuterium (1H2) is a stable, non-radioactive isotope that has one proton and one neutron. It occurs naturally at 0.0156%.
3) Tritium (1H3) is a radioactive isotope that has one proton and two neutrons. It occurs naturally at only 4.0 x 10-15%.
The document also discusses theTest for cat ions an ions
Test for cat ions an ionsShazeel Dhiffushi
Ėý
This document provides information on common cation and anion tests in chemistry. It lists several cations (aluminum, ammonium, calcium, copper, iron, zinc, lead) and their reactions when aqueous sodium hydroxide or ammonia is added. Precipitates formed are identified. It also lists several anions (carbonate, chloride, iodide, nitrate, sulfate) and their confirmatory chemical tests involving reactions like the formation of gases, precipitates with silver or barium salts, or color changes. The document serves as a reference for students to identify common metal cations and inorganic anions through characteristic chemical reactions.Chemistry formula list 2 (Examville.com)
Chemistry formula list 2 (Examville.com)JSlinkyNY
Ėý
1. The document provides information on the rates of chemical reactions including definitions and methods for calculating rates from graphs.
2. It also summarizes several common chemical reactions like precipitation, combustion, substitution, addition, oxidation and reduction reactions.
3. Details are given for important industrial processes like the Haber, Contact, and Ostwald processes for producing ammonia, sulfuric acid, and nitric acid respectively.Interfering radicals in qualitative analysis
Interfering radicals in qualitative analysisrajeeshRajeeshpraj
Ėý
Certain anions like oxalate, tartrate, fluoride, borate, phosphate, and chromate can interfere with the qualitative analysis of cations if not removed. They are eliminated through processes like dry ignition (oxalate), treatment with hydrochloric acid (fluoride, borate), precipitation with zirconyl nitrate (phosphate), and evaporation with hydrochloric acid (chromate). Arsenate is first reduced to arsenite using ammonium iodide before both are eliminated by precipitation with hydrogen sulfide. The order of elimination is oxalate, tartrate, fluoride, borate, phosphate, and arsenate/arsenite to ensure accurate analysis of metal cations.Qualitative analysis 1
Qualitative analysis 1Mark Selby
Ėý
The document outlines the curriculum and objectives for a qualitative analytical chemistry course at QUT, emphasizing the importance of chemical literacy and practical laboratory skills. It offers guidance on report writing, chemical nomenclature, and safe handling practices for acids and bases. Additionally, it includes information on various chemical compounds, their properties, and proper procedures for lab safety.Ppt2 Tests For Anions
Ppt2 Tests For Anionssitinurbaiyah
Ėý
This document describes methods to identify different anions through precipitation reactions. It lists the anions that can be identified as nitrate, sulfate, iodide, and carbonate. For each anion, it provides the reagent used to cause precipitation and the color of the precipitate formed. Identification is achieved by observing the precipitate color during reactions with specific reagents.3. qualitative analysis
3. qualitative analysisPluviose
Ėý
Qualitative analysis is used to identify the cations and anions present in an unknown chemical substance. Cations such as sodium, calcium, and ammonium can be identified using sodium hydroxide and ammonia solutions. Anions like chloride, nitrate, and sulfate can be identified through chemical tests involving silver nitrate, sodium hydroxide with aluminum foil, and barium chloride solutions respectively. These tests produce characteristic precipitates or gas emissions to reveal the ions present. Dilute nitric acid is first added to remove any interfering carbonate ions.Sodium hydroxide
Sodium hydroxidealanjoye
Ėý
Sodium hydroxide, also known as caustic soda, is a strong base with the chemical formula NaOH. It is a white crystalline solid that is highly soluble in water. Sodium hydroxide is an ionic compound composed of sodium cations and hydroxide anions, and it reacts strongly with acids to form salts and water through neutralization reactions. It also reacts with acidic gases like carbon dioxide to form less harmful compounds. Sodium hydroxide plays an important industrial role in processes like soap production through saponification reactions with fatty acids.Chemistry formula list 1 (Examville.com)
Chemistry formula list 1 (Examville.com)JSlinkyNY
Ėý
The document provides information on chemical formulae, equations, calculations involving moles and molar mass/volume. It also covers the chemical properties and reactions of group 1 and 17 elements, as well as properties of salts such as solubility, color, and the effects of heating on different salts such as carbonates and nitrates.IB Chemistry on Redox Titration, Biological Oxygen Demand and Redox.
IB Chemistry on Redox Titration, Biological Oxygen Demand and Redox.Lawrence kok
Ėý
This document discusses titration methods including acid-base titration and redox titration. It provides details on common primary standard acids and bases used in titration as well as indicators. It also discusses the principles and reactions involved in acid-base titration and redox titration. Examples are given of various redox titrations to determine concentrations of substances like copper, iron, chlorine, vitamin C, and more. Procedures and calculations for determining percentage compositions of substances from redox titrations are outlined.Acid base and salt class 10 science|| what are indicators
Acid base and salt class 10 science|| what are indicatorsPromilabis
Ėý
The document covers the properties and reactions of acids, bases, and salts, detailing how acids produce hydrogen ions in water, taste sour, and turn blue litmus red. It lists various reactions between acids and metals, carbonates, and bicarbonates, along with the characteristics of bases that are bitter and turn red litmus blue. The document also explains the role of indicators in identifying acids and bases, mentioning natural and synthetic indicators.Environmental issues of sodium hydroxide
Environmental issues of sodium hydroxidealanjoye
Ėý
Sodium hydroxide is a highly corrosive and irritating substance. It can cause severe burns upon contact with skin, eyes, or if ingested or inhaled. It reacts violently with metals like aluminum and with acids. If released into the environment, it can raise the pH of water and oceans, negatively impacting marine life. However, it may help neutralize atmospheric carbon dioxide by reacting to form sodium bicarbonate. Proper safety precautions must be taken when handling sodium hydroxide due to its hazardous properties.Silver nitrate
Silver nitrateZainab&Sons
Ėý
Silver nitrate is an inorganic compound with the chemical formula AgNO3. It has many uses including as an antiseptic, for treating warts, and cauterizing wounds. Silver nitrate can be prepared by reacting silver with nitric acid, producing silver nitrate and nitrogen oxide byproducts. It decomposes when heated, producing silver metal and oxygen and nitrogen gases. In solution, silver nitrate is used as a mild antiseptic and was historically used to prevent eye infections in newborns.7 h solutions (boardworks)
7 h solutions (boardworks)cartlidge
Ėý
The document is a chemistry textbook section about solutions. It introduces key concepts like mixtures, solutions, solutes and solvents. It describes different techniques for separating mixtures, including filtration, evaporation, distillation and chromatography. It also discusses how solubility is affected by temperature and saturation.Salt preparation by titration
Salt preparation by titrationjslayer
Ėý
This document describes the process of preparing salts through titration. It explains that titration allows the neutralization of an acid and base to be carried out exactly, producing a soluble salt. It provides the example of preparing sodium chloride through titrating hydrochloric acid with sodium hydroxide. The steps involve adding an indicator, titrating the acid with the base until the endpoint is reached, evaporating the solution to leave behind salt crystals, and filtering and drying the crystals. It asks how ammonium nitrate could be prepared using this method.Group 7 unit 1
Group 7 unit 1dean dundas
Ėý
This PowerPoint presentation covers group 7 (halogens) of the periodic table. It discusses trends in properties down the group such as appearance, boiling point, atomic radius, electronegativity, and oxidizing power. These trends are demonstrated through displacement reactions where a more reactive halogen will displace a less reactive one from a halide salt. Other reactions covered include the reaction of chlorine with water and alkalis. Methods for testing for halides using silver nitrate and concentrated sulfuric acid are also outlined.Qualitative analysis of anions
Qualitative analysis of anionsNIDHICH
Ėý
This document provides an overview of qualitative inorganic analysis for identifying anions present in compounds. It discusses preliminary and confirmatory tests for several common anions including carbonate, nitrite, acetate, sulfide, sulfite, chloride, bromide, iodide, nitrate, and oxalate. Tests involve observing reactions, such as gas evolution or precipitate formation, when the sample is treated with acids or reagents. Positive test results are indicated by characteristic observations that confirm the presence of the suspected anion.Applications of salts
Applications of saltsKapil Venkat
Ėý
This document discusses various types of salts and their properties and applications. It defines salts as ionic compounds that result from acid-base neutralization reactions. It provides examples of common salts like sodium chloride, potassium sulfate and lists their properties such as being crystalline solids with high melting/boiling points that dissolve in water and conduct electricity. It then describes specific applications of salts like sodium chloride in the Chlor-alkali process, baking soda in baking and antacids, and plaster of paris in supporting fractured bones.Lss acids and alkalis
Lss acids and alkalisJacklyn Kong
Ėý
This document discusses acids and alkalis. It defines acids as substances that produce hydrogen ions in water and alkalis as substances that produce hydroxide ions in water. Examples of common acids and alkalis are provided. The properties of acids and alkalis are described, including their sour taste, corrosiveness, and ability to conduct electricity. Their chemical reactions with metals, carbonates, and each other are outlined. Indicators are described as substances that change color in acids and alkalis to show their pH.Final not yet
Final not yetXixiViolet
Ėý
The document discusses transition metals chromium, molybdenum, and tungsten, which are grouped together based on their similar properties. It provides details on their physical properties like melting points, oxidation states, and natural resources. It also describes their chemical properties such as how they react with oxygen, halogens, and other substances. Their various compounds are discussed along with examples and reactions. Biological roles and applications are also covered.Types of clouds 1
Types of clouds 1nermine_ghis
Ėý
There are three main types of clouds: stratus clouds which are blanket-like, cumulus clouds which are billowy and puffy rising from a flat bottom, and cirrus clouds which are wispy and feather-like. Clouds can be named according to their altitude by using Latin prefixes like "cirro" for high altitude or "alto" for middle altitude clouds, or suffixes like "nimbus" to indicate storm clouds. Examples of named clouds include alto stratus clouds with the "alto" prefix and cumulo nimbus clouds with the "nimbus" suffix.Unit 2 Post-Assessment Review
Unit 2 Post-Assessment ReviewDkatrina76
Ėý
This document provides instructions for solving several math problems. It discusses finding the maximum or minimum of a function, and locating the axis of symmetry by finding the x-coordinate of the vertex.More Related Content
What's hot (20)
Test for cat ions an ions
Test for cat ions an ionsShazeel Dhiffushi
Ėý
This document provides information on common cation and anion tests in chemistry. It lists several cations (aluminum, ammonium, calcium, copper, iron, zinc, lead) and their reactions when aqueous sodium hydroxide or ammonia is added. Precipitates formed are identified. It also lists several anions (carbonate, chloride, iodide, nitrate, sulfate) and their confirmatory chemical tests involving reactions like the formation of gases, precipitates with silver or barium salts, or color changes. The document serves as a reference for students to identify common metal cations and inorganic anions through characteristic chemical reactions.Chemistry formula list 2 (Examville.com)
Chemistry formula list 2 (Examville.com)JSlinkyNY
Ėý
1. The document provides information on the rates of chemical reactions including definitions and methods for calculating rates from graphs.
2. It also summarizes several common chemical reactions like precipitation, combustion, substitution, addition, oxidation and reduction reactions.
3. Details are given for important industrial processes like the Haber, Contact, and Ostwald processes for producing ammonia, sulfuric acid, and nitric acid respectively.Interfering radicals in qualitative analysis
Interfering radicals in qualitative analysisrajeeshRajeeshpraj
Ėý
Certain anions like oxalate, tartrate, fluoride, borate, phosphate, and chromate can interfere with the qualitative analysis of cations if not removed. They are eliminated through processes like dry ignition (oxalate), treatment with hydrochloric acid (fluoride, borate), precipitation with zirconyl nitrate (phosphate), and evaporation with hydrochloric acid (chromate). Arsenate is first reduced to arsenite using ammonium iodide before both are eliminated by precipitation with hydrogen sulfide. The order of elimination is oxalate, tartrate, fluoride, borate, phosphate, and arsenate/arsenite to ensure accurate analysis of metal cations.Qualitative analysis 1
Qualitative analysis 1Mark Selby
Ėý
The document outlines the curriculum and objectives for a qualitative analytical chemistry course at QUT, emphasizing the importance of chemical literacy and practical laboratory skills. It offers guidance on report writing, chemical nomenclature, and safe handling practices for acids and bases. Additionally, it includes information on various chemical compounds, their properties, and proper procedures for lab safety.Ppt2 Tests For Anions
Ppt2 Tests For Anionssitinurbaiyah
Ėý
This document describes methods to identify different anions through precipitation reactions. It lists the anions that can be identified as nitrate, sulfate, iodide, and carbonate. For each anion, it provides the reagent used to cause precipitation and the color of the precipitate formed. Identification is achieved by observing the precipitate color during reactions with specific reagents.3. qualitative analysis
3. qualitative analysisPluviose
Ėý
Qualitative analysis is used to identify the cations and anions present in an unknown chemical substance. Cations such as sodium, calcium, and ammonium can be identified using sodium hydroxide and ammonia solutions. Anions like chloride, nitrate, and sulfate can be identified through chemical tests involving silver nitrate, sodium hydroxide with aluminum foil, and barium chloride solutions respectively. These tests produce characteristic precipitates or gas emissions to reveal the ions present. Dilute nitric acid is first added to remove any interfering carbonate ions.Sodium hydroxide
Sodium hydroxidealanjoye
Ėý
Sodium hydroxide, also known as caustic soda, is a strong base with the chemical formula NaOH. It is a white crystalline solid that is highly soluble in water. Sodium hydroxide is an ionic compound composed of sodium cations and hydroxide anions, and it reacts strongly with acids to form salts and water through neutralization reactions. It also reacts with acidic gases like carbon dioxide to form less harmful compounds. Sodium hydroxide plays an important industrial role in processes like soap production through saponification reactions with fatty acids.Chemistry formula list 1 (Examville.com)
Chemistry formula list 1 (Examville.com)JSlinkyNY
Ėý
The document provides information on chemical formulae, equations, calculations involving moles and molar mass/volume. It also covers the chemical properties and reactions of group 1 and 17 elements, as well as properties of salts such as solubility, color, and the effects of heating on different salts such as carbonates and nitrates.IB Chemistry on Redox Titration, Biological Oxygen Demand and Redox.
IB Chemistry on Redox Titration, Biological Oxygen Demand and Redox.Lawrence kok
Ėý
This document discusses titration methods including acid-base titration and redox titration. It provides details on common primary standard acids and bases used in titration as well as indicators. It also discusses the principles and reactions involved in acid-base titration and redox titration. Examples are given of various redox titrations to determine concentrations of substances like copper, iron, chlorine, vitamin C, and more. Procedures and calculations for determining percentage compositions of substances from redox titrations are outlined.Acid base and salt class 10 science|| what are indicators
Acid base and salt class 10 science|| what are indicatorsPromilabis
Ėý
The document covers the properties and reactions of acids, bases, and salts, detailing how acids produce hydrogen ions in water, taste sour, and turn blue litmus red. It lists various reactions between acids and metals, carbonates, and bicarbonates, along with the characteristics of bases that are bitter and turn red litmus blue. The document also explains the role of indicators in identifying acids and bases, mentioning natural and synthetic indicators.Environmental issues of sodium hydroxide
Environmental issues of sodium hydroxidealanjoye
Ėý
Sodium hydroxide is a highly corrosive and irritating substance. It can cause severe burns upon contact with skin, eyes, or if ingested or inhaled. It reacts violently with metals like aluminum and with acids. If released into the environment, it can raise the pH of water and oceans, negatively impacting marine life. However, it may help neutralize atmospheric carbon dioxide by reacting to form sodium bicarbonate. Proper safety precautions must be taken when handling sodium hydroxide due to its hazardous properties.Silver nitrate
Silver nitrateZainab&Sons
Ėý
Silver nitrate is an inorganic compound with the chemical formula AgNO3. It has many uses including as an antiseptic, for treating warts, and cauterizing wounds. Silver nitrate can be prepared by reacting silver with nitric acid, producing silver nitrate and nitrogen oxide byproducts. It decomposes when heated, producing silver metal and oxygen and nitrogen gases. In solution, silver nitrate is used as a mild antiseptic and was historically used to prevent eye infections in newborns.7 h solutions (boardworks)
7 h solutions (boardworks)cartlidge
Ėý
The document is a chemistry textbook section about solutions. It introduces key concepts like mixtures, solutions, solutes and solvents. It describes different techniques for separating mixtures, including filtration, evaporation, distillation and chromatography. It also discusses how solubility is affected by temperature and saturation.Salt preparation by titration
Salt preparation by titrationjslayer
Ėý
This document describes the process of preparing salts through titration. It explains that titration allows the neutralization of an acid and base to be carried out exactly, producing a soluble salt. It provides the example of preparing sodium chloride through titrating hydrochloric acid with sodium hydroxide. The steps involve adding an indicator, titrating the acid with the base until the endpoint is reached, evaporating the solution to leave behind salt crystals, and filtering and drying the crystals. It asks how ammonium nitrate could be prepared using this method.Group 7 unit 1
Group 7 unit 1dean dundas
Ėý
This PowerPoint presentation covers group 7 (halogens) of the periodic table. It discusses trends in properties down the group such as appearance, boiling point, atomic radius, electronegativity, and oxidizing power. These trends are demonstrated through displacement reactions where a more reactive halogen will displace a less reactive one from a halide salt. Other reactions covered include the reaction of chlorine with water and alkalis. Methods for testing for halides using silver nitrate and concentrated sulfuric acid are also outlined.Qualitative analysis of anions
Qualitative analysis of anionsNIDHICH
Ėý
This document provides an overview of qualitative inorganic analysis for identifying anions present in compounds. It discusses preliminary and confirmatory tests for several common anions including carbonate, nitrite, acetate, sulfide, sulfite, chloride, bromide, iodide, nitrate, and oxalate. Tests involve observing reactions, such as gas evolution or precipitate formation, when the sample is treated with acids or reagents. Positive test results are indicated by characteristic observations that confirm the presence of the suspected anion.Applications of salts
Applications of saltsKapil Venkat
Ėý
This document discusses various types of salts and their properties and applications. It defines salts as ionic compounds that result from acid-base neutralization reactions. It provides examples of common salts like sodium chloride, potassium sulfate and lists their properties such as being crystalline solids with high melting/boiling points that dissolve in water and conduct electricity. It then describes specific applications of salts like sodium chloride in the Chlor-alkali process, baking soda in baking and antacids, and plaster of paris in supporting fractured bones.Lss acids and alkalis
Lss acids and alkalisJacklyn Kong
Ėý
This document discusses acids and alkalis. It defines acids as substances that produce hydrogen ions in water and alkalis as substances that produce hydroxide ions in water. Examples of common acids and alkalis are provided. The properties of acids and alkalis are described, including their sour taste, corrosiveness, and ability to conduct electricity. Their chemical reactions with metals, carbonates, and each other are outlined. Indicators are described as substances that change color in acids and alkalis to show their pH.Final not yet
Final not yetXixiViolet
Ėý
The document discusses transition metals chromium, molybdenum, and tungsten, which are grouped together based on their similar properties. It provides details on their physical properties like melting points, oxidation states, and natural resources. It also describes their chemical properties such as how they react with oxygen, halogens, and other substances. Their various compounds are discussed along with examples and reactions. Biological roles and applications are also covered.Viewers also liked (6)
Types of clouds 1
Types of clouds 1nermine_ghis
Ėý
There are three main types of clouds: stratus clouds which are blanket-like, cumulus clouds which are billowy and puffy rising from a flat bottom, and cirrus clouds which are wispy and feather-like. Clouds can be named according to their altitude by using Latin prefixes like "cirro" for high altitude or "alto" for middle altitude clouds, or suffixes like "nimbus" to indicate storm clouds. Examples of named clouds include alto stratus clouds with the "alto" prefix and cumulo nimbus clouds with the "nimbus" suffix.Unit 2 Post-Assessment Review
Unit 2 Post-Assessment ReviewDkatrina76
Ėý
This document provides instructions for solving several math problems. It discusses finding the maximum or minimum of a function, and locating the axis of symmetry by finding the x-coordinate of the vertex.American economy
American economypj1005
Ėý
The American economy has the largest nominal GDP of $15 trillion and is a mixed economy influenced by its financial markets. It experienced the Great Depression in the 1930s and a financial crisis in 2007. The Federal Reserve, or FED, acts as the central bank and its chairman Ben Bernanke implemented Operation Twist to boost the economy and reduce unemployment. The Standard and Poor's 500 stock index rose 14% last quarter after a sell-off, and the FED's actions helped put it back on the rise, though full recovery is still uncertain.Copie de Message from tracesdegeometries@live....Danober
Ėý
Le document fournit un lien vers un fichier Google Docs. Il met en avant la facilitÃĐ de crÃĐation, de stockage et de partage de documents, tableurs et prÃĐsentations en ligne. Le message provient d'une adresse email spÃĐcifique.Fall 2011 Gaming Class Logistics
Fall 2011 Gaming Class LogisticsRyan Schaaf
Ėý
This document provides an overview of a course on gaming and media design for learning. The course explores learning theories behind game design and how commercial gaming technologies can be applied educationally. It brings together perspectives on developing and integrating electronic gaming to enhance learning. Students will develop an understanding of gaming trends and how insights from gaming can positively impact teaching and learning. The syllabus details assignments, expectations, resources needed, and due dates.BÎđÎŋΚÎŧÎđΞιÏÎđÎšÏ ÏÏÎŊÏÎđ
BÎđÎŋΚÎŧÎđΞιÏÎđÎšÏ ÏÏÎŊÏÎđAlex Paradissis
Ėý
ÎĪÎĩÏÎ―ÎŋÎŧÎŋÎģÎđΚÎŪ ÎĩÏÎģÎąÏÎŊÎą ΚιÏÎąÏΚÎĩÏ
ÎŪÏ ÎēÎđÎŋΚÎŧÎđΞιÏÎđΚÎŋÏ ÏÏÎđÏÎđÎŋÏAd
Similar to Kimia analisis kelompok 4 kelas 11.5 (9)
Iodometric and iodimetric Titration
Iodometric and iodimetric TitrationGreen University Of Bangladesh
Ėý
The document discusses iodometric and iodimetric titrations, which are analytical chemistry methods used to determine concentrations of unknown solutions. Iodometry involves indirect titration to estimate oxidizing agents, while iodimetry involves direct titration to quantify reducing agents. The document also highlights various applications of these methods, particularly in determining sulfites, hydrogensulfites, sulfides, and hydrogensulfides. Iodometric titration cannot be doine at higher pH.pdf
Iodometric titration cannot be doine at higher pH.pdfKARTIKINDIA
Ėý
Iodometric titration is not viable at high pH (>8) due to the conversion of iodine to hypoiodide and at low pH due to oxidation of iodide to iodine and interference with thiosulfate. The pH of the sample should be carefully adjusted before analysis as some reactions are reversible at specific pH levels. Measures such as ensuring excess iodide, avoiding light and contaminants, and using dry ice for prolonged titrations are recommended to prevent errors.Coulometry is an electrochemical hmethod
Coulometry is an electrochemical hmethodkom304299
Ėý
Coulometry is an electrochemical method in which the total charge (the number of coulombs) consumed in the redox conversion of an analyte at an electrode is measured. It is not to be confused with colorimetry, the spectroscopic methodCoulometry is an electrochemical hmethod
Coulometry is an electrochemical hmethodkom304299
Ėý
Coulometry is an electrochemical method in which the total charge (the number of coulombs) consumed in the redox conversion of an analyte at an electrode is measured. It is not to be confused with colorimetry, the spectroscopic methodOxidation reduction titration
Oxidation reduction titrationRajendra Patil
Ėý
This document discusses various types of redox titrations and indicators used. It describes the preparation and standardization of common redox titrants like potassium manganate(VII), iodine, potassium dichromate, potassium bromate and ceric ammonium sulfate. Examples of titrations included are standardization of KMnO4 with sodium oxalate or sodium thiosulfate, iodine with sodium thiosulfate or arsenic trioxide, and sodium thiosulfate with potassium iodate. The document also covers redox indicators and conditions for iodometric titrations.Oxidation reduction titration
Oxidation reduction titrationSuvarnaVanjari
Ėý
This document discusses various types of redox titrations and indicators used. It describes the preparation and standardization of common redox titrants like potassium manganate(VII), iodine, potassium dichromate, potassium bromate and ceric ammonium sulfate. Examples of titrations include standardization of iodine with sodium thiosulfate and arsenic trioxide, and standardization of sodium thiosulfate with potassium iodate. Precautions for different redox titrations and reactions involved are also explained.redox titration B pharm 1st sem Full notes
redox titration B pharm 1st sem Full notesSantosh kumar
Ėý
The document provides an overview of redox titrations, including definitions, types, and applications. It explains the concepts of oxidation and reduction, different redox indicators, and specific methods such as iodimetry, iodometry, cerimetry, and dichromate titrations. Additionally, it highlights the importance of redox titrations in various fields such as pharmaceutical analysis, industrial testing, and environmental monitoring.PA-I Redox titration.(HRB)
PA-I Redox titration.(HRB)Harshadaa bafna
Ėý
This document discusses redox titrations. It begins by defining oxidation and reduction reactions. It then discusses different types of redox titrations including cerimetry, iodimetry, iodometry, bromatometry, dichrometry, and titration with potassium iodate. For each type of titration, the document describes the basic principles and provides some examples of applications. The document is presented by Miss Harshada R. Bafna and contains information on concepts, types, and specific techniques for various redox titration methods.PA I - Redox Titration Pharmaceutical analysis
PA I - Redox Titration Pharmaceutical analysisSheebaElavarasi1
Ėý
Redox titration is a laboratory technique for determining the concentration of analytes through oxidation-reduction reactions, involving a titrant and an analyte. Key components include oxidizing and reducing agents, with various types of titrations like iodometry, bromatometry, cerimetry, permanganometry, and dichrometry, each utilizing specific methods and indicators for detection. The document details the processes, preparations, standardizations, and applications of these titrations in analytical chemistry.Ad
Kimia analisis kelompok 4 kelas 11.5
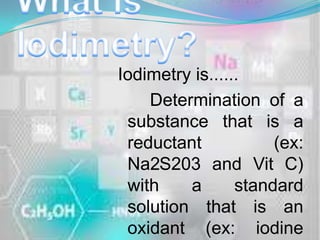
- 3. Iodimetry is...... Determination of a substance that is a reductant (ex: Na2S203 and Vit C) with a standard solution that is an oxidant (ex: iodine
- 4. reaction, because in this titration we used I2 for titrat who has character oxidator. Iodimetry can produce (terbatas) some substance. Indicator can be used: 1. Amylum 1% 2. CCl4 Iodimetri Titration performed in netral condition or weak acid in the range up to a weak base
- 5. Iodometri consist of 2 is : A. Iodimetri direct method Bahan pereduksi langsung dioksidasi dengan larutan baku Iodium. Contohnya pada penetapan kadar Asam Askorbat. B. Iodimetri metode residual ( titrasi balik) Bahan pereduksi dioksidasi dengan larutan baku iodium dalam jumlah berlebih, dan kelebihan iod akan dititrasi dengan larutan baku natrium tiosulfat. Contohnya pada penetapan kadar Natrium Bisulfit.
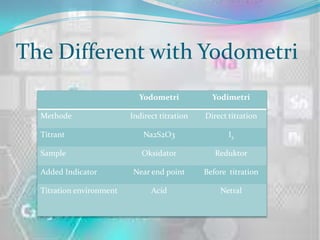
- 6. The Different with Yodometri Yodometri Yodimetri Methode Indirect titration Direct titration Titrant Na2S2O3 I2 Sample Oksidator Reduktor Added Indicator Near end point Before titration Titration environment Acid Netral
- 7. The advantages : âĒFaster Processing âĒTA is easily to observation Disadvantages : âĒEasly evaporate âĒI2 is volatile
- 8. pH of Reaction Titrasi iodimetri dilakukan dalam keadaan netral atau dalam kisaran asam lemah sampai basa lemah. Pada pH tinggi (basa kuat) maka iodine dapat mengalami reaksi disproporsionasi menjadi hipoiodat. I2 + 2OH- IO3- + I- + H2O Sedangkan pada keadaan asam kuat maka amilum yang dipakai sebagai indicator akan terhidrolisis, selain itu pada keadaan ini iodide (I-) yang dihasilkan dapat diubah menjadi I2 dengan adanya O2 dari udara bebas, reaksi ini melibatkan H+ dari asam. 4I- + O2 + 4H+ 2I2 + 2H2O
- 9. âĒ Oxidation level -1,+1,+3,+5, dan +7 âĒ Weak oxidator âĒ Can be reacted with all of metal element and non metal element âĒ Can be reacted with Hidrogen fomed Halide acid âĒ Can formed an Oxy acid with formula HIO,HIO2,HIO3,HIO4.
- 10. âĒ The solid, black sparkle bluish âĒ Volatile gas at room temperature into a blue violet with a pungent odor âĒ Easly soluble chloroform, carbon tetrachloride, or carbon disulfide âĒ Slightly soluble in water âĒ properties of metal resembling âĒ May cause mumps
- 12. A ( Reduktor ) + I2 â A ( Teroksidasi ) + 2 I -
- 13. âĒ Add 18 grams of KI âĒ Dissolve in water âĒ Weight 12.90 g I2 âĒ Dissolve in water âĒ Pour KI solution into I2 solution âĒ Dilute to 1 L
- 14. âĒ Weighed 1.0000 grams As2O3 + âĒ Introduced into 100 ml measuring flask âĒ Added + 10 ml 4N NaOH âĒ Added 2-3 drops of PP âĒ Neutralized with 4N H2SO4, until the pink color of the solution becomes a hint âĒ Added 3 grams of crystal NaHCO3 âĒ Pipette 10 ml, inserted into erlenmeyer teaser, then diluted to 100ml + âĒ Added indicator Kanji 2 -3 drops âĒ Dititar with I2 solution until it reaches TA Blue light hint
