Latest Studies in Cancer Immunotherapy - Shauket Hossain.pptx
- 1. SHAUKET HOSSAIN Deputy General Manager, Marketing UniHealth Limited, Bangladesh Latest Studies in Cancer Immunotherapy
- 2. Agenda ’ü▒ Brief idea on cancer immunotherapies ’ü▒ Update on currently available cancer immunotherapies and their approved indications ’ü▒ Outline of key information and results of five latest clinical studies on different cancer immunotherapies
- 3. Birth of cancer immunotherapy Ref. Immunotargets Ther. 2018 Apr 23;7:29-34. ’ü▒ Dr. William Bradley Coley Has been regarded as the founder of cancer immunotherapy for his legendary discovery of ŌĆśErysipelas Germs as Cure for CancerŌĆÖ in 1908.
- 4. Cancer immunotherapy Ref. Int J Cardiol. 2021 Jan 15;323:179-187. ’ü▒ Immunotherapy is a type of targeted cancer treatment that helps our immune system to fight cancer. ’ü▒ It is a biological therapy made from living organisms or cells. ’ü▒ It helps the immune system to fight against cancer in a better way. ’ü▒ The goal of immunotherapy is to achieve durable tumor regression & possible cure with minimum adverse effects on patients.
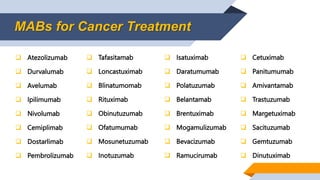
- 5. MABs for Cancer Treatment ’ü▒ Atezolizumab ’ü▒ Durvalumab ’ü▒ Avelumab ’ü▒ Ipilimumab ’ü▒ Nivolumab ’ü▒ Cemiplimab ’ü▒ Dostarlimab ’ü▒ Pembrolizumab ’ü▒ Tafasitamab ’ü▒ Loncastuximab ’ü▒ Blinatumomab ’ü▒ Rituximab ’ü▒ Obinutuzumab ’ü▒ Ofatumumab ’ü▒ Mosunetuzumab ’ü▒ Inotuzumab ’ü▒ Isatuximab ’ü▒ Daratumumab ’ü▒ Polatuzumab ’ü▒ Belantamab ’ü▒ Brentuximab ’ü▒ Mogamulizumab ’ü▒ Bevacizumab ’ü▒ Ramucirumab ’ü▒ Cetuximab ’ü▒ Panitumumab ’ü▒ Amivantamab ’ü▒ Trastuzumab ’ü▒ Margetuximab ’ü▒ Sacituzumab ’ü▒ Gemtuzumab ’ü▒ Dinutuximab
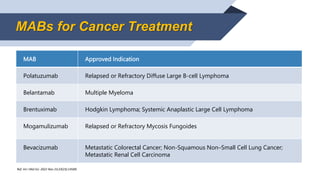
- 6. Ref. Int J Mol Sci. 2022 Nov 23;23(23):14589. MABs for Cancer Treatment MAB Approved Indication Atezolizumab Non-Small Cell Lung Cancer; Extensive-Stage Small Cell Lung Cancer; Unresectable or Metastatic Hepatocellular Carcinoma; Unresectable or Metastatic Melanoma Durvalumab Non-Small Cell Lung Cancer; Extensive-Stage Small Cell Lung Cancer; Unresectable or Metastatic Hepatocellular Carcinoma; Locally Advanced or Metastatic Biliary Tract Cancer Avelumab Metastatic Merkel Cell Carcinoma; Urothelial Carcinoma Ipilimumab Metastatic Melanoma; Advanced Renal Cell Carcinoma; Non-Small Cell Lung Cancer; Colorectal Carcinoma
- 7. MABs for Cancer Treatment MAB Approved Indication Nivolumab Metastatic Melanoma; Metastatic Non-Small Cell Lung Cancer; Small Cell Lung Cancer; Advanced Renal Cell Carcinoma; Classical Hodgkin Lymphoma; Squamous Cell Carcinoma of Head & Neck; Urothelial Carcinoma; Metastatic Colorectal Cancer; Hepatocellular Carcinoma Cemiplimab Metastatic Cutaneous Squamous Cell Carcinoma; Locally Advanced Cutaneous Squamous Cell Carcinoma; Non-small Cell Lung Cancer Dostarlimab Advanced Endometrial Cancer Pembrolizumab Advanced Melanoma; Advanced NonŌüĀŌĆōŌüĀSmall Cell Lung Cancer; Relapsed or Refractory Classical Hodgkin Lymphoma; Refractory or Relapsed Mediastinal Large B-cell Lymphoma; Advanced Urothelial Carcinoma; High-Risk Non-muscle Invasive Bladder Cancer; High-Risk Risk Early-Stage Triple-Negative Breast Cancer
- 8. Ref. Int J Mol Sci. 2022 Nov 23;23(23):14589. MABs for Cancer Treatment MAB Approved Indication Tafasitamab Refractory Diffuse Large B-cell Lymphoma Loncastuximab Relapsed or Refractory Large B-cell Lymphoma Blinatumomab B-precursor Acute Lymphoblastic Leukemia Rituximab Non-Hodgkin Lymphoma, Chronic Lymphocytic Leukemia Obinutuzumab Chronic Lymphocytic Leukemia; Follicular Lymphoma
- 9. Ref. Int J Mol Sci. 2022 Nov 23;23(23):14589. MABs for Cancer Treatment MAB Approved Indication Ofatumumab Relapsing forms of Multiple Sclerosis Mosunetuzumab Relapsed or Refractory Follicular Lymphoma Inotuzumab Relapsed or Refractory B-cell Precursor Acute Lymphoblastic Leukemia Isatuximab Multiple Myeloma Daratumumab Multiple Myeloma
- 10. Ref. Int J Mol Sci. 2022 Nov 23;23(23):14589. MABs for Cancer Treatment MAB Approved Indication Polatuzumab Relapsed or Refractory Diffuse Large B-cell Lymphoma Belantamab Multiple Myeloma Brentuximab Hodgkin Lymphoma; Systemic Anaplastic Large Cell Lymphoma Mogamulizumab Relapsed or Refractory Mycosis Fungoides Bevacizumab Metastatic Colorectal Cancer; Non-Squamous NonŌĆōSmall Cell Lung Cancer; Metastatic Renal Cell Carcinoma
- 11. Ref. Int J Mol Sci. 2022 Nov 23;23(23):14589. MABs for Cancer Treatment MAB Approved Indication Trastuzumab Adjuvant Breast Cancer; Metastatic Breast Cancer; Metastatic Gastric Cancer Sacituzumab Metastatic Triple-negative Breast Cancer Gemtuzumab Newly-Diagnosed CD33-positive Acute Myeloid Leukemia; Relapsed or Refractory CD33-positive AML Dinutuximab Pediatric Patients with High-risk Neuroblastoma
- 12. Latest Studies in Cancer Immunotherapy
- 13. Methodology ’ü▒ This presentation outlines the key information and results of five latest clinical studies on different cancer immunotherapies. ’ü▒ The studies are selected through a search in PubMed (Keyword: ŌĆśCancer ImmunotherapyŌĆÖ; Sorted by: ŌĆśMost RecentŌĆÖ; Filter: ŌĆśClinical TrialŌĆÖ and ŌĆśPublication time 1 yearŌĆÖ). ’ü▒ Only randomized controlled studies are included here.
- 14. KEYNOTE-716 Study ’ü▒ Double-blind, randomized, placebo-controlled, Phase-3 study ’ü▒ 160 academic medical centers & hospitals in 16 countries (Australia, Belgium, Brazil, Canada, Chile, France, Germany, Israel, Italy, Japan, Poland, South Africa, Spain, Switzerland, UK & USA) ’ü▒ 976 patients with stage IIB or IIC melanoma who underwent surgery were enrolled ’ü▒ Patients received either 200 mg pembrolizumab (n=487) of or saline placebo (n=489) intravenously every 3 weeks for 17 cycles ’ü▒ Median follow-up: 27.4 months Ref. Lancet. 2022 Apr 30;399(10336):1718-1729.
- 15. KEYNOTE-716 Study Ref. Lancet. 2022 Apr 30;399(10336):1718-1729. ’ü▒ Pembrolizumab as adjuvant therapy in stage IIB or IIC melanoma patients resulted in a significant reduction in the risk of disease recurrence or death versus placebo, with a manageable safety profile
- 16. KEYNOTE-564 Study ’ü▒ Double-blind, randomized, placebo-controlled, Phase-3 study ’ü▒ 213 hospitals & cancer centers in North America, South America, Europe, Asia, and Australia ’ü▒ 994 patients with renal cell carcinoma with an increased risk of recurrence were enrolled ’ü▒ Patients received either 200 mg (n=496) of or saline placebo (n=498) intravenously every 3 weeks for 17 cycles ’ü▒ Median follow-up: 30.1 months Ref. Lancet Oncol. 2022 Sep;23(9):1133-1144.
- 17. KEYNOTE-564 Study ’ü▒ Disease-free survival was better with pembrolizumab compared with placebo Ref. Lancet Oncol. 2022 Sep;23(9):1133-1144. HR 0.63 [95% CI 0.50-0.80]
- 18. ORIENT-11 Study ’ü▒ Double-blind, randomized, placebo-controlled, Phase-3 study ’ü▒ Conducted in Chinese participants from mainland China ’ü▒ 397 patients with treatment-na├»ve advanced metastatic non- squamous NSCLC were enrolled ’ü▒ Patients received either Sintilimab + Pemetrexed-Platinum (n=266) or Placebo + Pemetrexed-Platinum (n=131) ’ü▒ Median follow-up: 30.8 months Ref. Lung Cancer. 2022 Sep;171:56-60.
- 19. ORIENT-11 Study Ref. Lung Cancer. 2022 Sep;171:56-60. ’ü▒ Median OS (Overall Survival) was 24.2 months in Sintilimab arm & 16.8 months in placebo arm ’ü▒ OS was better in Sintilimab arm (HR:0.65, 95 % CI, 0.50-0.85).
- 20. ORIENT-31 Study ’ü▒ Double-blind, randomized, placebo-controlled, Phase-3 study ’ü▒ Conducted at 52 hospitals in China ’ü▒ 444 patients with locally advanced or metastatic EGFR-mutated NSCLC were enrolled ’ü▒ Patients received either Sintilimab + Bevacizumab + Chemotherapy (n=148) or Sintilimab + Chemotherapy (n=145) or Chemotherapy alone (n=151) ’ü▒ Median follow-up: 9.8 months Ref. Lancet Oncol. 2022 Sep;23(9):1167-1179.
- 21. ORIENT-31 Study Ref. Lancet Oncol. 2022 Sep;23(9):1167-1179. ’ü▒ Progression-free survival was significantly longer in Sintilimab + Bevacizumab + Chemotherapy group versus Chemotherapy alone group (HR 0.46, 0.34-0.64; p<0.0001).
- 22. CAPSTONE-1 Study ’ü▒ Double-blind, randomized, placebo-controlled, Phase-3 study ’ü▒ Conducted at 47 tertiary hospitals in China ’ü▒ 462 patients with extensive-stage small-cell lung cancer were enrolled ’ü▒ Patients received either Adebrelimab + Chemotherapy (n=230) or Placebo + Chemotherapy (n=232) ’ü▒ Median follow-up: 13.5 months Ref. Lancet Oncol. 2022 Sep;23(9):1167-1179.
- 23. CAPSTONE-1 Study Ref. Lancet Oncol. 2022 Sep;23(9):1167-1179. ’ü▒ Median overall survival was significantly improved in the Adebrelimab group (HR 0.72, 95% CI, 0.58-0.90; p=0.0017)
- 24. Summary # Study PatientŌĆÖs Information Treatment Protocol Key Result 1. KEYNOTE-716 976 patients with stage IIB or IIC melanoma who underwent surgery Pembrolizumab (n=487) or Placebo (n=489) Median follow-up: 27┬Ę4 months ’éĘ Pembrolizumab significantly improved metastasis-free survival (HR 0┬Ę64, 95% CI, 0┬Ę47-0┬Ę88; p=0┬Ę0029) versus placebo. 2. KEYNOTE-564 994 patients with clear cell RCC who underwent surgery Pembrolizumab (n=496) or Placebo (n=498) Median follow-up: 30.1 months ’éĘ Disease-free survival was better with pembrolizumab (HR 0┬Ę63, 95% CI, 0┬Ę50-0┬Ę80). 3. ORIENT-11 397 patients with treatment- na├»ve advanced metastatic nonsquamous NSCLC Sintilimab + Pemetrexed-Platinum (n=266) or Placebo + Pemetrexed-Platinum (n=131) Median follow-up: 30.8 months ’éĘ Overall survival was better in Sintilimab arm (HR:0.65, 95 % CI, 0.50-0.85). 4. ORIENT-31 444 patients with locally advanced or metastatic EGFR-mutated NSCLC Sintilimab + Bevacizumab + Chemotherapy (n=148) or Sintilimab + Chemotherapy (n=145) or Chemotherapy alone (n=151) Median follow-up: 9.8 months ’éĘ Progression-free survival was significantly longer in the Sintilimab + Bevacizumab + Chemotherapy group versus the Chemotherapy alone group (HR 0┬Ę46, 0┬Ę34-0┬Ę64; p<0┬Ę0001). 5. CAPSTONE-1 462 patients with extensive- stage small-cell lung cancer Adebrelimab + Chemotherapy (n=230) or Placebo + Chemotherapy (n=232) Median follow-up: 13┬Ę5 months ’éĘ Median overall survival was significantly improved in the Adebrelimab group (HR 0┬Ę72, 95% CI, 0┬Ę58-0┬Ę90; p=0┬Ę0017)
- 25. THANKS!
Editor's Notes
- #2: A very good morning to all of you. Before starting my presentation, I would like to express my heartfelt gratitude & sincere thanks to Larix International for inviting me. Today, I am going to discuss with you regarding the ŌĆśLatest Studies in Cancer ImmunotherapyŌĆÖ. To be precise, from this prestationŌĆ”
- #3: ŌĆ”first you will get a brief idea on cancer immunotherapies; then the update on currently available cancer immunotherapies and their approved indications. Finally, you will have a clear outline of key information & results of five latest clinical studies on different cancer immunotherapies. I hope this presentation would help you to select the right immunotherapy for the right patient on the basis of the latest clinical evidences. So, letsŌĆÖ start with right from the beginning that is the birth of cancer immunotherapy.
- #4: More than 115 years ago, in 1908, Dr. William Bradley Coley first reported Erysipelas Germs as Cure for Cancer. Which was published in New York Times at time. In fact, this is the starting cancer immunotherapy. Hence, Dr. William Bradley Coley Has been regarded as the founder of cancer immunotherapy. Afterwards, cancer immunotherapy has been enriched with many more life saving innovations. Now from 1908, letsŌĆÖ back in again in todayŌĆÖs scenario and see more details on cancer immunotherapy.
- #5: Immunotherapy is a type of targeted cancer treatment that helps our immune system to fight cancer. It is a biological therapy made from living organisms or cells. It helps the immune system to fight against cancer in a better way. The goal of╠²immunotherapy is to achieve durable╠²tumor regression & possible cure with minimum adverse effects on patients. We can classify the immunotherapies in six broad classes: 1. monoclonal antibodies 2. check-point inhibitors 3. bi-specific T-cell engagers 4. cytokines 5. anti-cancer vaccines 6. cart T-cells. Among them, monoclonal antibodies or MABs are most widely used. Hence, we will limit our following discussion on MABs only.














![KEYNOTE-564 Study
’ü▒ Disease-free
survival was better
with
pembrolizumab
compared with
placebo
Ref. Lancet Oncol. 2022 Sep;23(9):1133-1144.
HR 0.63 [95% CI 0.50-0.80]](https://image.slidesharecdn.com/lateststudiesincancerimmunotherapy-shaukethossain-240127114709-adeb71d9/85/Latest-Studies-in-Cancer-Immunotherapy-Shauket-Hossain-pptx-17-320.jpg)























































