Presentatio suji
This document discusses problems with evidence from clinical drug studies and why such evidence may fail to accurately predict a drug's clinical utility. Some key issues include: insufficient consideration of all available evidence; use of weak study designs like non-randomized trials; industry sponsorship resulting in bias; lack of head-to-head comparisons; choice of populations and outcomes that do not reflect real-world use; publication and reporting biases that suppress negative results; and reliability issues with meta-analyses and clinical guidelines formed from potentially biased evidence. Solutions proposed include improving study registration and data sharing, requiring comparative evidence earlier, and conducting reviews in a more transparent and non-conflicted manner.















Recommended




























































More Related Content
What's hot (20)










































Viewers also liked (12)






























Similar to Presentatio suji (20)


























![[Inf 295] week 6 parul seth patient-reported outcomes as a source of evidence...](https://cdn.slidesharecdn.com/ss_thumbnails/inf295week6parulsethpatient-reportedoutcomesasasourceofevidenceinoff-labelprescribing-131107002627-phpapp01-thumbnail.jpg?width=560&fit=bounds)
![[Inf 295] week 6 parul seth patient-reported outcomes as a source of evidence...](https://cdn.slidesharecdn.com/ss_thumbnails/inf295week6parulsethpatient-reportedoutcomesasasourceofevidenceinoff-labelprescribing-131107002627-phpapp01-thumbnail.jpg?width=560&fit=bounds)














Presentatio suji
- 1. How Good Is ˇ°Evidenceˇ± From Clinical Studies Of Drug Effects and Why Might Such Evidence Fail in the Prediction of the Clinical Utility of Drugs? O (Naci H.,Ioannidis P.A.,Annu.Rev.Pharmacol.Toxicol.,2015,55:169-189) 1 SUJITA MISHRA PI/289
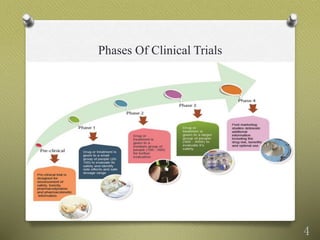
- 4. Phases Of Clinical Trials 4
- 5. Problems in the Design of Clinical Studies of Drug Effects Insufficient Considerations Of Evidence Use Of Weak Study Designs Industry Sponsorship Of Research Lack Of Head¨Cto-Head Comparisons Choice Of Patient Population Choice Of Outcomes 5
- 6. Insufficient Considerations of Evidence Continuously Updated Assessments Published Reports May Not Cited Focus On Consulting Reviews Disproportionate research 6
- 7. Use Of Weak Study Designs NON-RANDOMIZED DESIGNS RANDOMIZED DESIGNS 7
- 8. Industry Sponsorship Of Research Most Clinical Drug Research Is Sponsored by Pharmaceutical Industry Industry Sponsored Studies Favour the Products Funded by Other Sources 8
- 9. Lack Of Head-to-Head Comparisons 9
- 10. Choice Of Patient Populations 10
- 11. Choice Of Outcomes Predict the Real-World Utility It Includes Composites Of Multiple Outcomes 11
- 12. Problems in the Reporting of Clinical Trials PUBLICATION BIAS REPORTING PAUCITY OF EVIDENCE ON HARMS 12
- 13. Publication Generally Reported Positive Results Unpublished Trials Are Now Posted In ClinicalTrials.gov. 13
- 14. Bias Reporting Selective Citation of Clinical Studies Selective Outcome Reporting 14
- 15. Problems in the Evidence and in the Formation of Recommendations from Studies of Drug Effects METAANALYSES RELIABILITY OF CLINICAL PRACTICE GUIDELINES 15
- 16. Solution of Clinical TrialˇŻs Problems 16 Potential Improvements Study Registrations Provision Of Raw Data
- 17. Solution of Clinical TrialˇŻs Problems 17 Sponsoring of Clinical Research by Nonconflicted Entities Requirement of Comparative Data at the Time of Drug Approvals Nonconflicted Conduct Of Systematic Reviews
- 18. Future Aspects ENSURE TRANSPARENCY MEANINGFUL AND ACCURATE EVIDENCE DEVELOP TRANSFORMATIVE IDEAS ENHANCE PUBLIC TRUST 18
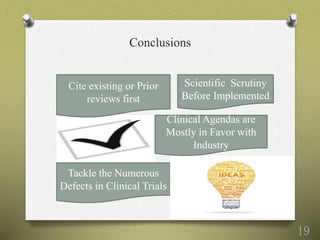
- 19. Conclusions 19 Cite existing or Prior reviews first Clinical Agendas are Mostly in Favor with Industry Tackle the Numerous Defects in Clinical Trials Scientific Scrutiny Before Implemented
- 20. Acknowledgments O I wish to acknowledge to- O Dr.SACHCHIDANAND SIR, O Dr.M.CHOURASIA SIR, O Dr.SHAILENDRA SIR, O SOHNI MAM, and O MY CLASSMATES. O I THANK YOU ALLˇˇ 20
- 21. 21
![[Week4] Google refine](https://cdn.slidesharecdn.com/ss_thumbnails/week4googlerefinelecture-150830004221-lva1-app6892-thumbnail.jpg?width=560&fit=bounds)