Presentazione12 (1).pptx
- 1. La radioattività DALLA SCOPERTA FINO AI GIORNI NOSTRI Di Alessandro Gianfelice V^D
- 2. Cosa si intende per radioattività ? Con il termine radioattività si intende quell'inisieme di processi chimico-fisici tramite cui alcuni nuclei atomici si trovano decadere per diventare più stabili. Tutti quegli elementi che posseggono un nucleo atomico instabile e quindi sono maggiormente susciettibili a tali processi sono chiamati elementi radioattivi.
- 3. La scoperta della radioattività naturale La radioattività naturale venne scoperta nel 1896 dal fisico Henri Becquerel il quale riteneva che i materiali fluorescenti sui quali faceva esperimenti erano in grado di produrre radioazioni diverse dal visibile, tra cui anche raggi x. Tramite esperimento egli riusci a impressionare su una lastra coperta da un involucro nero le radiazioni emesse da sali di uranio fluorescenti, anche senza che questi fossero esposti alla luce del sole. Egli così dimostrò che determinati elementi erano in grado di emettere radiazioni senza essere eccitati. Le radiazioni emesse da questi corpi vennero poi chiamate dall'allieva di Becquerel, Marie Curie, "radiazioni Becquerel".
- 4. Il contributo di Marie Curie Marie Curie, due volte premio nobel (nel 1903 per la fisica e nel 1911 per la chimica), è conosciuta per I suoi intensi studi sulle radiazioni. Ella, insieme al marito Pierre, analizzò diversi elementi e scoprì che l'uranio non era l'unico materiale a emettere radiazioni ma ne scoprirono diversi altri. Un altro elemento ad esempio era il torio. Ai due si deve la scoperta inoltre di due nuovi elementi chimici, ovvero il polonio e il radio, entrambi radioattivi.
- 5. Il decadimento radioattivo Il processo tramite il quale un atomo perde progressivamente particelle dal suo nucleo per diventare più stabile è detto decadimento radioattivo. Ne esistono in particolar modo tre tipi: • decadimento alfa; • decadimento beta; • decadimento gamma.
- 6. Caratteristiche delle radiazioni alfa, beta e gamma • Per studiare la natura e le caratteristiche delle radiazioni emesse dagli elementi radioattivi si utilizza un campo magnetico che deflette il moto delle particelle emesse se queste posseggono carica. In tal modo è possibile affermare che I raggi alfa sono composti da protoni, I raggi beta da elettroni e I raggi gamma da particelle neutre, che più in avanti si scoprirà essere neutroni. • Le tre radiazioni non sono ugualmente energetiche: I raggi alfa fanno difficoltà a oltrepassare anche solo pochi millimetri di carta; I raggi beta sono maggiormente penetranti ma si possono fermare anche davanti a qualche centimetro d'acqua; per arrestare le radiazioni gamma è necessario invece un materiale composto da elementi molto pesanti, ad esempio una lastra di piombo di alcuni centimetri.
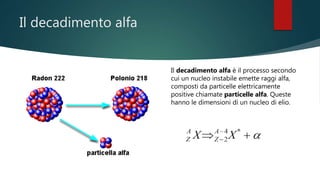
- 7. Il decadimento alfa Il decadimento alfa è il processo secondo cui un nucleo instabile emette raggi alfa, composti da particelle elettricamente positive chiamate particelle alfa. Queste hanno le dimensioni di un nucleo di elio.
- 8. Il decadimento gamma Nel decadimento gamma un nucleo instabile passa da uno stato di eccitazione a quello fondamentale tramite l'emissione di radiazioni gamma. Sono in assoluto le più energetiche e di conseguenza le più nocive.
- 9. Il decadimento beta Il decadimento beta è il processo secondo cui un nucleo instabile emette elettroni o positroni. Esistono quindi due tipi di decadimento beta: • Beta negativo; • Beta positivo.
- 10. I problemi del decadimento beta Fino alla scoperta del neutrone non si riusciva a spiegare come un nucleo atomico, il quale non era composto da elettroni, potesse emetterne durante i processi di decadimento beta. Si ipotizzò che degli elettroni potessero essere presenti in maniera permanente nel nucleo ma questa interpretazione andava contro il principio di conservazione dell'energia. Qualcosa non quadrava, sia nella struttura del nucleo sia nelle ipotesi del decadimento beta. Inoltre il decadimento beta sembrava violare anche il principio di conservazione del momento angolare e il principio di indeterminazione di Heisenberg.
- 11. L'ipotesi del neutrino Un altro problema del decadimento beta è legato alla conservazione della quantità di moto del sistema nucleo-elettrone. Infatti se il nucleo di partenza è fermo, il nucleo figlio e l'elettrone secondo tale principio dovrebbero muoversi con la stessa quantità di moto in direzioni opposte. In tal modo sarebbe facile anche calcolare l'energia cinetica dei due. Tuttavia i calcoli e i risultati sperimentali differiscono di una certa quantità : l'energia trasportata dall'elettrone è solo una parte di quella prodotta. Doveva esistere dunque un'altra particella, di carica neutra, che trasportava la restante energia. Fu così che nel 1930 Wolfgang Pauli ipotizzò l'esistenza di tale particella cui Enrico Fermi diede il nome di neutrino.
- 12. La scoperta del neutrone Nel 1932 James Chadwick riesce, con la scoperta del neutrone, a descrivere la formazione del nucleo dell'atomo e a risolvere molti dei problemi legati al decadimento beta. Egli riprese alcuni esperimenti effettuati da Walter Bothe, Richard Becker e Irene Joliot Curie (figlia di Marie Curie) i quali verificarono che le particelle alfa emesse dal Polonio, incidendo su Berillio, producevano una radiazione capace di superare 200 mm di Piombo; per cui era necessario capire la natura di questa radiazione, che Chadwick scoprì essere costituita da particelle neutre di massa pressoché simile a quella del protone.
- 13. Il settimo Congresso Solvay In questo contesto le domande che riguardavano il decadimento beta erano quasi esclusivamente legate a come venissero prodotti elettroni e neutrini e che ruolo giocasse il neutrone in tal processo. Il tema era fortemente dibattuto all'interno del settimo Congresso Solvay, una conferenza tenutasi nel 1933 cui vari scienziati e fisici di tutto il mondo si riunirono per discutere di questi problemi.
- 14. Il contributo di Enrico Fermi L'unico scienziato italiano che partecipò al settimo Congresso Solvay fu Enrico Fermi. Premio nobel nel 1938 grazie ai suoi studi sulle reazioni nucleari innescate dai neutroni lenti, egli rimase affascinato dalle ipotesi di Pauli sul neutrino e sul decadimento beta. Egli ipotizzò che, come quando un elettrone eccitato passa da un livello più energetico a uno meno energetico esso emette un fotone, anche durante il decadimento beta il neutrino e l'elettrone vengono creati istantaneamente. Per dimostrare tale teoria vennero impiegati molti artifici matematici. Grazie a questa tutte le domande riguardanti il decadimento beta ottennero risposta.
- 15. L'attività radioattiva Con attività di un oggetto radioattivo si intende il numero di decadimenti al secondo che avvengono in esso. Il numero di nuclei attivi N con il tempo diminuisce; per cui si può scrivere che l'attività è uguale al rapporto tra la variazione del numero di nuclei e la variazione temporale in cui avviene il decadimento. Questo è inoltre uguale al prodotto tra il numero di nuclei iniziale e la costante di decadimento. La formula che esprime il numero di nuclei a un dato istante di tempo è:
- 16. Il tempo di dimezzamento e la vita media • Il tempo di dimezzamento di un materiale radioattivo è il tempo che viene impiegato affinché il numero di nuclei all'interno dell'oggetto si dimezzi. Sfruttando la relazione di prima è possibile verificare che il prodotto tra il tempo di dimezzamento e la costante di decadimento del materiale è uguale a ln(2). • La vita media di un nucleo radioattivo è il tempo medio necessario affinché un nucleo decada ed è uguale al reciproco della costante di decadimento del materiale.
- 17. Conseguenze delle radiazioni ionizzanti Le radiazioni emesse dai materiali radioattivi sono comprese in quelle che vengono definite radiazioni ionizzanti. Queste sono molto nocive per l'essere umano in quanto sono sufficientemente energetiche da liberare gli elettroni dall'atomo per cui possono fare sì che si verifichino danni a livello del genoma. La stessa Marie Curie è morta in seguito alla forte e prolungata esposizione alle radiazioni per tempi estremamente lunghi.
- 18. Applicazioni dei materiali radioattivi nell'archelogia La radioattività è stata impiegata in numerosi modi ai giorni nostri. Uno degli esempi più lampanti di applicazione dei principi della radioattività sono le radiazioni radiometriche. Se un oggetto contiene nuclei radioattivi al momento della sua formazione, il tempo di decadimento può essere utilizzato per determinare l'età del campione.