Shapiro reaction
Download as PPTX, PDF13 likes12,568 views
This document discusses the Shapiro reaction, which was discovered by Robert H. Shapiro in 1967. The reaction involves converting aryl sulfonyl hydrazones of aldehydes and ketones into olefins using alkyl lithium reagents, grignard reagents, or alkali metal amides at -78°C. The reaction mechanism proceeds through deprotonation, elimination, and loss of nitrogen to form alkenyl intermediates. The Shapiro reaction has been used in the total synthesis of natural products like phytocassane D and in the formation of ring B in the Nicolaou Taxol total synthesis.
1 of 18
Downloaded 102 times
![SHAPIRO REACTION
[TOSYL HYDRAZONE DECOMPOSITION]
SUBMITTED BY :
RINSHANA FATHIMA ABDUL AZEEZ
FIRST YEAR M.PHARM
PHARMACEUTICAL CHEMISTRY
AL SHIFA COLLEGE OF PHARMACY
1](https://image.slidesharecdn.com/shapiroreaction-190218212126/85/Shapiro-reaction-1-320.jpg)
![âĒ Discovered by Robert H. Shapiro in 1967
âĒ Shapiro reaction (1967) involves the conversion of aryl sulfonyl
hydrazones of aldehydes and ketones into olefins by reacting
with alkyl lithium reagent, grignard reagent, or alkali metal
amide.[1]
âĒ This reaction is carried out at a temperature of -78â
2](https://image.slidesharecdn.com/shapiroreaction-190218212126/85/Shapiro-reaction-2-320.jpg)
![The reaction similar to Bamford Stevens reaction where bases such
as Na, NaOMe, LiH, NaH, NaNH2, etc. are used.
Another major difference between the two reactions is that Shapiro
reaction yields less substitued olefins as kinetcic products (the
product that predominates when the reaction is done at low
temperature.) While Bamford Stevens reaction yields more
substituted olefins as the thermodynamic product (the product that
predominates when the reaction is done at high temperature.)[2]
3](https://image.slidesharecdn.com/shapiroreaction-190218212126/85/Shapiro-reaction-3-320.jpg)

![MECHANISM
1. Deprotonation of -NH proton from aryl sulfonyl hydrazone.
2. Second deprotonation adjacent to the hydrazone group to give
a dianion.
3. Elimination of toluene sulfinate to form an intermediate
(carbanion mechanism).
4. Loss of N2 to form alkenylithium species which is then
protonated (treated with an electrophile) to give olefins.[1]
6](https://image.slidesharecdn.com/shapiroreaction-190218212126/85/Shapiro-reaction-6-320.jpg)
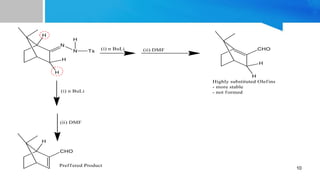
![âĒ Synthesis of an allylic alchol, which is an intermediate in the synthesis of
Mequitazine, Which is an H1 anti histaminic used to treat allergies and
rhinitis. [5]
12
SYNTHETIC APPLICATIONS](https://image.slidesharecdn.com/shapiroreaction-190218212126/85/Shapiro-reaction-12-320.jpg)
![âĒ (-)-phytocassane D was synthesized from (R)-Wieland Miescher Ketone
by this reaction, for determining the absolute configuration of the
phytocassane group of the phytoalexins.[3]
13
WMK is a racemic bicyclic
diketone used in the total
synthesis of more than 50 natural
compounds](https://image.slidesharecdn.com/shapiroreaction-190218212126/85/Shapiro-reaction-13-320.jpg)
![âĒ Shapiro reaction is involved in the formation of ring B in the Nicolaou
Taxol total synthesis.[4]
A
B
D
C
14](https://image.slidesharecdn.com/shapiroreaction-190218212126/85/Shapiro-reaction-14-320.jpg)
![âĒ A class of Chiral indenes (Verbindenes) was prepared from Verbenone
by utilising Shapiro reaction as one of the key transformation step.[3]
15](https://image.slidesharecdn.com/shapiroreaction-190218212126/85/Shapiro-reaction-15-320.jpg)



Recommended
Suzuki reaction



Suzuki reactionRinshana Fathima
Ėý
The Suzuki reaction is a palladium-catalyzed cross-coupling reaction between boronic acids or esters with organic halides, triflates, or other boron-containing compounds. This reaction occurs under basic conditions and leads to the formation of carbon-carbon single bonds, typically between an aryl or vinyl group and another aryl or vinyl group. It is commonly used to synthesize biaryl compounds. The reaction proceeds through oxidative addition, transmetallation, and reductive elimination steps. Key advantages are mild reaction conditions and availability of boronic acids. The Suzuki reaction has applications in synthesizing pharmaceuticals, agrochemicals, and natural products.Suzuki and Shapiro reaction 



Suzuki and Shapiro reaction Shalinee Chandra
Ėý
The document summarizes the Suzuki and Shapiro reactions. The Suzuki reaction involves a palladium-catalyzed cross-coupling between organoboron compounds and organic halides to form carbon-carbon bonds. It proceeds through oxidative addition, transmetallation, and reductive elimination steps. The Shapiro reaction involves the base-catalyzed decomposition of tosyl hydrazones to form olefins. Both reactions have been used in the synthesis of various drugs and natural products.Wiliknsons reagent



Wiliknsons reagentShikha Popali
Ėý
Wilkinson's catalyst, also known as chloridotris(triphenylphosphane)rhodium(I), is a coordination complex of rhodium with the formula RhCl(PPh3)3. It is a red-brown solid that is soluble in hydrocarbon solvents and used widely as a catalyst for hydrogenation of alkenes. Wilkinson's catalyst is obtained by treating rhodium(III) chloride hydrate with excess triphenylphosphine, which acts as a reducing agent to reduce rhodium from Rh(III) to Rh(I). It adopts a slightly distorted square planar structure and undergoes fast dynamic exchange processes in solution.Mannich reaction



Mannich reactionAkshay Sharma
Ėý
The Mannich reaction involves the condensation of an enolizable carbonyl compound, an aldehyde such as formaldehyde, and an amine to form a Îē-amino carbonyl compound known as a Mannich base. The reaction proceeds via the initial addition of the amine to the aldehyde to form an iminium ion intermediate, which then reacts with the enol form of the carbonyl compound to eliminate a proton and form the Mannich base product. While versatile building blocks in organic synthesis, the Mannich reaction has limitations in terms of substrate scope and control of regio- and stereoselectivity. Examples of applications include the synthesis of tropinone, a precursor of atropine, as wellMichael addition reaction 



Michael addition reaction AkshithaDn
Ėý
Michael addition reaction involves the conjugate addition of a nucleophile to an Îą,Îē-unsaturated carbonyl compound. It proceeds through a reversible 1,2-addition of the nucleophile to the Îē-carbon, which can then undergo irreversible proton elimination to form the more stable thermodynamic enolate product by removing the Ï-bond of the C=C. This reaction is useful for forming C-C bonds and natural products, with examples given of its application in multi-step syntheses using further carbonyl reactions like aldol condensation.Dicyclohexylcarbodiimide



DicyclohexylcarbodiimideDepartment of Pharmaceutical Science and Natural Products, central University of Punjab
Ėý
Dicyclohexylcarbodiimide (DCC) is an organic compound that is commonly used as a synthetic reagent to couple amino acids during peptide synthesis. It was first introduced for this purpose in 1955. DCC is a waxy white solid with a sweet odor that is highly soluble in organic solvents but insoluble in water. It has a low melting point, which allows it to be easily melted and handled. DCC is commonly used to catalyze esterification reactions and form amide bonds, as well as synthesize peptides, ethers, acid anhydrides, and lactones. One of its key applications is in the synthesis of beta-lactam rings in penicillin.Brook rearrangement



Brook rearrangementAnusreeAnu11
Ėý
It is an intramolecular rearrangement reaction in which the 1,2-migration of silyl group from carbon to oxygen under basic conditions.It involves the formation of a pentacoordinate siliconintermediate.Discovered by Adrian Gibbs Brook in 1958.Heck reaction



Heck reactionHarbansh Singhal
Ėý
The document introduces the Heck reaction, which is a coupling reaction where a metal catalyst aids in coupling two hydrocarbon fragments. Specifically, the Heck reaction involves converting a terminal alkene to an internal alkene. Richard Heck, Ei-ichi Negishi, and Akira Suzuki were jointly awarded the Nobel Prize in 2010 for their work developing palladium-catalyzed C-C cross coupling reactions, including the Heck reaction. The mechanism of the Heck reaction involves oxidative addition, insertion, Îē-H elimination, and reductive elimination steps.DISCONNECTION-retrosynthesis.pptx



DISCONNECTION-retrosynthesis.pptxHimani Kolekar
Ėý
An approach for designing organic synthesis which involves breaking down of target molecule into available starting material by imaginary breaking of bonds (disconnection) and/or by functional group interconversion is known as disconnection approach or retrosynthesis or synthesis backward.
The C-X disconnection approach is mainly applicable to a carbon chain attached to any of the heteroatoms like O, N, or S. Here, a bond joins the heteroatom (X) to the rest of the molecule like a C-O, C-N, or C-S group. This point is good point to initiate a disconnection. This is called a âOne-groupâ C-X disconnection as one would need to identify only one functional group like ester, ether, amide etc. to make the disconnection.
How to choose a disconnection?
These are the few general strategy which are important points introduced which apply to the whole of synthetic design rather than one particular area. The main choice is between the various disconnection, even such a simple disconnection as the following alcohol can be disconnected.
We want to get back to simple starting materials and we shall do if we disconnect the bond which are:
Towards the middle of the molecule thereby breaking into two reasonably equal halves rather than chopping off one or two carbon atoms from the end and,
At a branch as this is more likely to give straight chain fragments and these are more likely to be available.
Disconnections very often take place immediately adjacent to, or very close to functional groups in the target molecule. This is pretty much inevitable, given that functionality almost invariably arises from the forward reaction.
A simple example is the weedkiller propanil used on rice fields. Amide disconnection gives amine obviously made from o-dichlorobenzene by nitration and reduction. All positions around the ring in o-dichlorobenzene are about the same electronically but steric hindrance will lead to dichloronitrobenzene being the major product
This compound was needed for some research into the mechanisms of rearrangements. We can disconnect on either side of the ether oxygen atom, but (b) is much better because (a) does not correspond to a reliable reaction: it might be hard to control selective alkylation of the primary hydroxyl group in the presence of the secondary one.
The disconnections we have made so far have all been of CâO, CâN, or CâS bonds, but, of course, the most important reactions in organic synthesis are those that form CâC bonds. We can analyze CâC disconnections in much the same way as weâve analyzed CâX disconnections.
The Zeneca drug propranolol is a beta-blocker that reduces blood pressure and is one of the top drugs worldwide. It has two 1,2-relationships in its structure but it is best to disconnect the more reactive amine group first.
Arildone is a drug that prevents polio and herpes simplex viruses from âunwrappingâ their DNA, and renders them harmless.Protection for carboxylic group & Protection for the Amino group



Protection for carboxylic group & Protection for the Amino groupCollege of Pharmacy,Sri Ramakrishna Institute of Paramedical Sciences,Coimbatore
Ėý
Protecting groups are introduced to temporarily block reactive sites on molecules to allow for selective reactivity in multi-step organic syntheses. Common protecting groups include esters for carboxylic acids (e.g. methyl esters), acetals for carbonyls, and alkyl/acyl/sulfonyl groups for amines. Protecting groups must be stable but also removable, without affecting other functionality. For example, a ketone might be protected as an acetal to allow for reduction of an ester without reducing the ketone as well.Ullmann reaction 



Ullmann reaction Aanchal Gupta
Ėý
The Ullmann reaction involves the condensation of aryl halides in the presence of finely divided copper or copper bronze at an elevated temperature to form diaryl derivatives. Two proposed mechanisms are the free radical mechanism, where copper generates an aryl radical, and the ionic mechanism, where an organocuprate intermediate is formed. The Ullmann reaction is useful for synthesizing biaryls, polyaryls, diaryl amines, diaryl ethers, and gossypol.Ultrasound



UltrasoundSuraj Patil
Ėý
This document summarizes an ultrasound assisted reaction presentation. It discusses how ultrasound differs from conventional energy sources and how it can be used in organic synthesis and green and pharmaceutical chemistry. It describes how sonochemistry works through cavitation, where bubbles form and violently collapse, generating high pressures and temperatures. This can enhance chemical reactivity in homogeneous liquid, heterogeneous solid/liquid, and heterogeneous liquid/liquid phase reactions. Examples of synthetic applications where ultrasound switching altered reaction pathways are provided. The conclusion discusses how bubble collapse concentrates energy that can be used to heat bubble contents and enhance reactivity.Nitration (2)



Nitration (2)Nehapriya32
Ėý
it contains all the subtopic of the topic nitration that is to be studied in M.pharm pharmaceutical chemistry Synthetic reagent and applications OF ALUMINIUM ISOPROPOXIDE



Synthetic reagent and applications OF ALUMINIUM ISOPROPOXIDEShikha Popali
Ėý
SYNTHETIC REAGENTS AND APPLICATIONS OF ALUMINIUM ISOPROPOXIDE ITS ALTERNATIVE NAMES AND ITS PHYSICAL PROPERTIRS , HANDLING, STORAGE, PRECAUTIONS, PREPARATIONS, SYNTHETIC APPLICATIONS Reactions of heterocyclic chemistry



Reactions of heterocyclic chemistrysuraj wanjari
Ėý
This document summarizes several organic reactions used in heterocyclic chemistry. It describes the DebusâRadziszewski reaction for imidazole synthesis, the Knorr reaction for pyrrole synthesis, the Pinner reaction for pyrimidine synthesis, the Combes reaction for quinoline synthesis, the Bernthsen reaction for acridine synthesis, the Smiles rearrangement, and the Traube reaction for purine synthesis. For each reaction, it provides the starting materials, product, mechanism, and some applications. The document is intended to present an overview of important heterocyclic reactions for students of pharmaceutical chemistry.Wagnor meerwin reaction



Wagnor meerwin reactionwadhava gurumeet
Ėý
The document discusses the Wagner-Meerwein rearrangement, a reaction first observed in 1899 where a carbocation is generated followed by a [1,2]-shift of an adjacent carbon-carbon bond to form a new carbocation. This reaction was not fully understood until 1922 when its ionic nature was revealed. The rearrangement involves the migration of hydrogen, alkyl, or aryl groups between carbocations and can involve multiple consecutive shifts. It can be initiated through various means to generate the initial carbocation and the migrating group retains its stereochemistry.Strategies for Heterocycle ring synthesis 



Strategies for Heterocycle ring synthesis SACHINKUMARVISHWAKAR4
Ėý
This document discusses strategies for synthesizing three, four, five, and six-membered heterocyclic rings. It outlines three strategies for each ring size, including the Gabriel ring closure and Hassner synthesis for aziridines, pyrolysis of cyclopropyl azides and photocycloaddition for azetines, the Paal-Knorr and Hantzsch syntheses for pyrroles, and the Hantzsch synthesis and reactions with maleic anhydride for pyridines and pyridazines. A variety of heterocyclic compounds are derived from carbocyclic precursors by replacing carbon atoms with heteroatoms like nitrogen, oxygen, or sulfur.Michael addition reaction 



Michael addition reaction Diwan Thakur
Ėý
The document discusses the Michael addition reaction, which involves the nucleophilic addition of a carbanion to an Îą,Îē-unsaturated carbonyl compound. It provides the definition, mechanism, examples including the synthesis of warfarin, and applications such as asymmetric Michael reactions. The mechanism involves deprotonation of the carbonyl compound by a base to form an enolate ion, which adds to the Michael acceptor to form a new carbon-carbon bond via 1,4-addition.organic reaction



organic reactionNiketBajare
Ėý
The document summarizes two organic reactions: the Dieckmann reaction and ozonolysis reaction. The Dieckmann reaction involves the intramolecular condensation of diesters in the presence of a strong base to form Îē-keto esters via a 5-exo-trig cyclization. It is used to synthesize cyclopentane and cyclohexane derivatives. Ozonolysis involves the cleavage of unsaturated bonds like alkenes and alkynes with ozone to form carbonyl groups. It can be used to oxidize alkenes into alcohols, aldehydes, ketones or carboxylic acids and is useful for structure elucidation of unknown compounds containing carbon-carbon double bonds.Protecting groups



Protecting groupsaqsaakram15
Ėý
This document discusses the use of protecting groups in organic synthesis. It provides examples of common protecting groups for alcohols, including trialkylsilyl ethers, benzyl ethers, and acetate esters. Methods for introducing and removing these protecting groups are described. The document also discusses protecting groups for amines, such as Boc and phthaloyl, along with their introduction and removal conditions. Finally, examples of acetal and ketal protecting groups for carbonyl compounds are briefly mentioned.Retro synthesis



Retro synthesisgopinathannsriramachandraeduin
Ėý
The document discusses retrosynthesis, which is the process of working backward from a target organic compound to develop a synthetic route. It involves imagining the cleavage of bonds to form synthons, which are idealized fragments represented as ions. Key steps in retrosynthesis are disconnection, which breaks bonds, and functional group interconversion, which changes one functional group to another. The goal is to develop the most efficient synthesis using readily available starting materials and reagents.4. Wilkinson's Catalyst



4. Wilkinson's CatalystShivendra Singh
Ėý
General characteristics, application and mechanism of Wilkinson's catalyst is discussed in the lecture.Mannich reaction



Mannich reactionAanchal Gupta
Ėý
The Mannich reaction involves the condensation of an enolizable carbonyl compound, an amine or ammonia, and formaldehyde to form an aminomethyl derivative known as a Mannich base. Ketones are most commonly used as the carbonyl compound. The reaction proceeds via the generation of an imine intermediate from the carbonyl compound and amine, which then reacts with formaldehyde to form the Mannich base. Mannich bases have applications in synthesizing natural products like alkaloids and building ring systems.PHASE TRANSFER CATALYSIS [PTC] ![PHASE TRANSFER CATALYSIS [PTC]](https://cdn.slidesharecdn.com/ss_thumbnails/64-191219161253-thumbnail.jpg?width=560&fit=bounds)
![PHASE TRANSFER CATALYSIS [PTC]](https://cdn.slidesharecdn.com/ss_thumbnails/64-191219161253-thumbnail.jpg?width=560&fit=bounds)
![PHASE TRANSFER CATALYSIS [PTC]](https://cdn.slidesharecdn.com/ss_thumbnails/64-191219161253-thumbnail.jpg?width=560&fit=bounds)
![PHASE TRANSFER CATALYSIS [PTC]](https://cdn.slidesharecdn.com/ss_thumbnails/64-191219161253-thumbnail.jpg?width=560&fit=bounds)
PHASE TRANSFER CATALYSIS [PTC] Shikha Popali
Ėý
PTC IS THE PHASE TRANSFER CATALYSIS HERE TYPES OF PTC ARE DISCUSSED , THEORIES OF CATALYSIS AND MECHANISM OF PTC, ADVANTAGES OF PTC, APPLICATION OF PTCPinner pyrimidine synthesis



Pinner pyrimidine synthesisJACOB THON BIOR
Ėý
This document summarizes the Pinner pyrimidine synthesis reaction. The reaction involves the condensation of a non-N-substituted amidine and Îē-keto ester (Îē-diketone) in the presence of an acid catalyst to form a pyrimidine heterocyclic ring. The mechanism proceeds through protonation, nucleophilic attack, dehydration, and deprotonation steps. Pyrimidine derivatives synthesized via this reaction are used in drugs to treat conditions like malaria, viruses, and cancer.SYNTHON APPROACH



SYNTHON APPROACHShikha Popali
Ėý
THE SYNTHON APPROACH DECRIBED IN THIS PRESENTATION DETAIL ACCOUNT ON SYNETHESIS WITH STRUCTURE, BASIC RULES, APPLICATIONS.ultrasound assisted reaction 2.pptx



ultrasound assisted reaction 2.pptxTahminaKhan20
Ėý
This document discusses types of sonochemical reactions, specifically heterogeneous solid/liquid and liquid/liquid reactions, and their synthetic applications. Heterogeneous solid/liquid reactions involve solids dispersed in liquids, where ultrasound enhances reactivity by cleaning the solid surface and increasing its effective area through cavitation bubble implosions and microstreaming. Heterogeneous liquid/liquid reactions generate emulsions that increase the interfacial contact area between immiscible liquids, allowing reactions to occur more readily across phase boundaries. Several examples of synthetic applications of these sonochemical reactions are provided, including esterification, saponification, Cannizzaro reactions, and Ullmann-type coupling reactions, which see increased yields, lower temperatures, or shorter reactionHeterocyclic compounds



Heterocyclic compoundspriyaswain27
Ėý
The document discusses several heterocyclic compounds including quinolines, isoquinolines, and indoles. It summarizes key reactions used to synthesize these compounds, including the Combes, Friedlander, Knorr, and Skraup reactions for quinoline synthesis. It also discusses the Bischler-Napieralski, Pictet-Spengler, and Pomeranz-Fritsch reactions for isoquinoline synthesis and the Fischer, Madelung, and Reissert reactions for indole synthesis, along with mechanisms and examples of each reaction. Reactivity and substitution patterns are also covered for quinolines, isoquinolines and indoles.Aromatic rearrangements



Aromatic rearrangementsMISHUSINGH1
Ėý
The document discusses several aromatic rearrangement reactions including the Fries rearrangement, photo-Fries rearrangement, Reimer-Tiemann reaction, Gattermann-Koch reaction, Gattermann aldehyde synthesis, and Vilsmeier-Haack reaction. The Fries rearrangement involves the rearrangement of phenolic esters to ortho- and para-substituted phenolic ketones/aldehydes using Lewis or BrÃļnsted acids. The photo-Fries rearrangement is similar but uses light instead of acids. The Reimer-Tiemann, Gattermann-Koch, and Vilsmeier-Haack reactions all involve the formylation of aromatic compounds to produceImportant Name reaction 



Important Name reaction Home
Ėý
Name reactions:Clemmensen reduction, Darzens condensation, Diels Alder, Eschweiler-Clarke, Friedel-Crafts, Grinard, HofmanMannich, Michael, Meerwin- Pondorf- Verley, Perkin, Reformatsky, Reimer-Tiemann,, Witting and Wolf-Kishner reduction, Aldol condensation.
More Related Content
What's hot (20)
DISCONNECTION-retrosynthesis.pptx



DISCONNECTION-retrosynthesis.pptxHimani Kolekar
Ėý
An approach for designing organic synthesis which involves breaking down of target molecule into available starting material by imaginary breaking of bonds (disconnection) and/or by functional group interconversion is known as disconnection approach or retrosynthesis or synthesis backward.
The C-X disconnection approach is mainly applicable to a carbon chain attached to any of the heteroatoms like O, N, or S. Here, a bond joins the heteroatom (X) to the rest of the molecule like a C-O, C-N, or C-S group. This point is good point to initiate a disconnection. This is called a âOne-groupâ C-X disconnection as one would need to identify only one functional group like ester, ether, amide etc. to make the disconnection.
How to choose a disconnection?
These are the few general strategy which are important points introduced which apply to the whole of synthetic design rather than one particular area. The main choice is between the various disconnection, even such a simple disconnection as the following alcohol can be disconnected.
We want to get back to simple starting materials and we shall do if we disconnect the bond which are:
Towards the middle of the molecule thereby breaking into two reasonably equal halves rather than chopping off one or two carbon atoms from the end and,
At a branch as this is more likely to give straight chain fragments and these are more likely to be available.
Disconnections very often take place immediately adjacent to, or very close to functional groups in the target molecule. This is pretty much inevitable, given that functionality almost invariably arises from the forward reaction.
A simple example is the weedkiller propanil used on rice fields. Amide disconnection gives amine obviously made from o-dichlorobenzene by nitration and reduction. All positions around the ring in o-dichlorobenzene are about the same electronically but steric hindrance will lead to dichloronitrobenzene being the major product
This compound was needed for some research into the mechanisms of rearrangements. We can disconnect on either side of the ether oxygen atom, but (b) is much better because (a) does not correspond to a reliable reaction: it might be hard to control selective alkylation of the primary hydroxyl group in the presence of the secondary one.
The disconnections we have made so far have all been of CâO, CâN, or CâS bonds, but, of course, the most important reactions in organic synthesis are those that form CâC bonds. We can analyze CâC disconnections in much the same way as weâve analyzed CâX disconnections.
The Zeneca drug propranolol is a beta-blocker that reduces blood pressure and is one of the top drugs worldwide. It has two 1,2-relationships in its structure but it is best to disconnect the more reactive amine group first.
Arildone is a drug that prevents polio and herpes simplex viruses from âunwrappingâ their DNA, and renders them harmless.Protection for carboxylic group & Protection for the Amino group



Protection for carboxylic group & Protection for the Amino groupCollege of Pharmacy,Sri Ramakrishna Institute of Paramedical Sciences,Coimbatore
Ėý
Protecting groups are introduced to temporarily block reactive sites on molecules to allow for selective reactivity in multi-step organic syntheses. Common protecting groups include esters for carboxylic acids (e.g. methyl esters), acetals for carbonyls, and alkyl/acyl/sulfonyl groups for amines. Protecting groups must be stable but also removable, without affecting other functionality. For example, a ketone might be protected as an acetal to allow for reduction of an ester without reducing the ketone as well.Ullmann reaction 



Ullmann reaction Aanchal Gupta
Ėý
The Ullmann reaction involves the condensation of aryl halides in the presence of finely divided copper or copper bronze at an elevated temperature to form diaryl derivatives. Two proposed mechanisms are the free radical mechanism, where copper generates an aryl radical, and the ionic mechanism, where an organocuprate intermediate is formed. The Ullmann reaction is useful for synthesizing biaryls, polyaryls, diaryl amines, diaryl ethers, and gossypol.Ultrasound



UltrasoundSuraj Patil
Ėý
This document summarizes an ultrasound assisted reaction presentation. It discusses how ultrasound differs from conventional energy sources and how it can be used in organic synthesis and green and pharmaceutical chemistry. It describes how sonochemistry works through cavitation, where bubbles form and violently collapse, generating high pressures and temperatures. This can enhance chemical reactivity in homogeneous liquid, heterogeneous solid/liquid, and heterogeneous liquid/liquid phase reactions. Examples of synthetic applications where ultrasound switching altered reaction pathways are provided. The conclusion discusses how bubble collapse concentrates energy that can be used to heat bubble contents and enhance reactivity.Nitration (2)



Nitration (2)Nehapriya32
Ėý
it contains all the subtopic of the topic nitration that is to be studied in M.pharm pharmaceutical chemistry Synthetic reagent and applications OF ALUMINIUM ISOPROPOXIDE



Synthetic reagent and applications OF ALUMINIUM ISOPROPOXIDEShikha Popali
Ėý
SYNTHETIC REAGENTS AND APPLICATIONS OF ALUMINIUM ISOPROPOXIDE ITS ALTERNATIVE NAMES AND ITS PHYSICAL PROPERTIRS , HANDLING, STORAGE, PRECAUTIONS, PREPARATIONS, SYNTHETIC APPLICATIONS Reactions of heterocyclic chemistry



Reactions of heterocyclic chemistrysuraj wanjari
Ėý
This document summarizes several organic reactions used in heterocyclic chemistry. It describes the DebusâRadziszewski reaction for imidazole synthesis, the Knorr reaction for pyrrole synthesis, the Pinner reaction for pyrimidine synthesis, the Combes reaction for quinoline synthesis, the Bernthsen reaction for acridine synthesis, the Smiles rearrangement, and the Traube reaction for purine synthesis. For each reaction, it provides the starting materials, product, mechanism, and some applications. The document is intended to present an overview of important heterocyclic reactions for students of pharmaceutical chemistry.Wagnor meerwin reaction



Wagnor meerwin reactionwadhava gurumeet
Ėý
The document discusses the Wagner-Meerwein rearrangement, a reaction first observed in 1899 where a carbocation is generated followed by a [1,2]-shift of an adjacent carbon-carbon bond to form a new carbocation. This reaction was not fully understood until 1922 when its ionic nature was revealed. The rearrangement involves the migration of hydrogen, alkyl, or aryl groups between carbocations and can involve multiple consecutive shifts. It can be initiated through various means to generate the initial carbocation and the migrating group retains its stereochemistry.Strategies for Heterocycle ring synthesis 



Strategies for Heterocycle ring synthesis SACHINKUMARVISHWAKAR4
Ėý
This document discusses strategies for synthesizing three, four, five, and six-membered heterocyclic rings. It outlines three strategies for each ring size, including the Gabriel ring closure and Hassner synthesis for aziridines, pyrolysis of cyclopropyl azides and photocycloaddition for azetines, the Paal-Knorr and Hantzsch syntheses for pyrroles, and the Hantzsch synthesis and reactions with maleic anhydride for pyridines and pyridazines. A variety of heterocyclic compounds are derived from carbocyclic precursors by replacing carbon atoms with heteroatoms like nitrogen, oxygen, or sulfur.Michael addition reaction 



Michael addition reaction Diwan Thakur
Ėý
The document discusses the Michael addition reaction, which involves the nucleophilic addition of a carbanion to an Îą,Îē-unsaturated carbonyl compound. It provides the definition, mechanism, examples including the synthesis of warfarin, and applications such as asymmetric Michael reactions. The mechanism involves deprotonation of the carbonyl compound by a base to form an enolate ion, which adds to the Michael acceptor to form a new carbon-carbon bond via 1,4-addition.organic reaction



organic reactionNiketBajare
Ėý
The document summarizes two organic reactions: the Dieckmann reaction and ozonolysis reaction. The Dieckmann reaction involves the intramolecular condensation of diesters in the presence of a strong base to form Îē-keto esters via a 5-exo-trig cyclization. It is used to synthesize cyclopentane and cyclohexane derivatives. Ozonolysis involves the cleavage of unsaturated bonds like alkenes and alkynes with ozone to form carbonyl groups. It can be used to oxidize alkenes into alcohols, aldehydes, ketones or carboxylic acids and is useful for structure elucidation of unknown compounds containing carbon-carbon double bonds.Protecting groups



Protecting groupsaqsaakram15
Ėý
This document discusses the use of protecting groups in organic synthesis. It provides examples of common protecting groups for alcohols, including trialkylsilyl ethers, benzyl ethers, and acetate esters. Methods for introducing and removing these protecting groups are described. The document also discusses protecting groups for amines, such as Boc and phthaloyl, along with their introduction and removal conditions. Finally, examples of acetal and ketal protecting groups for carbonyl compounds are briefly mentioned.Retro synthesis



Retro synthesisgopinathannsriramachandraeduin
Ėý
The document discusses retrosynthesis, which is the process of working backward from a target organic compound to develop a synthetic route. It involves imagining the cleavage of bonds to form synthons, which are idealized fragments represented as ions. Key steps in retrosynthesis are disconnection, which breaks bonds, and functional group interconversion, which changes one functional group to another. The goal is to develop the most efficient synthesis using readily available starting materials and reagents.4. Wilkinson's Catalyst



4. Wilkinson's CatalystShivendra Singh
Ėý
General characteristics, application and mechanism of Wilkinson's catalyst is discussed in the lecture.Mannich reaction



Mannich reactionAanchal Gupta
Ėý
The Mannich reaction involves the condensation of an enolizable carbonyl compound, an amine or ammonia, and formaldehyde to form an aminomethyl derivative known as a Mannich base. Ketones are most commonly used as the carbonyl compound. The reaction proceeds via the generation of an imine intermediate from the carbonyl compound and amine, which then reacts with formaldehyde to form the Mannich base. Mannich bases have applications in synthesizing natural products like alkaloids and building ring systems.PHASE TRANSFER CATALYSIS [PTC] ![PHASE TRANSFER CATALYSIS [PTC]](https://cdn.slidesharecdn.com/ss_thumbnails/64-191219161253-thumbnail.jpg?width=560&fit=bounds)
![PHASE TRANSFER CATALYSIS [PTC]](https://cdn.slidesharecdn.com/ss_thumbnails/64-191219161253-thumbnail.jpg?width=560&fit=bounds)
![PHASE TRANSFER CATALYSIS [PTC]](https://cdn.slidesharecdn.com/ss_thumbnails/64-191219161253-thumbnail.jpg?width=560&fit=bounds)
![PHASE TRANSFER CATALYSIS [PTC]](https://cdn.slidesharecdn.com/ss_thumbnails/64-191219161253-thumbnail.jpg?width=560&fit=bounds)
PHASE TRANSFER CATALYSIS [PTC] Shikha Popali
Ėý
PTC IS THE PHASE TRANSFER CATALYSIS HERE TYPES OF PTC ARE DISCUSSED , THEORIES OF CATALYSIS AND MECHANISM OF PTC, ADVANTAGES OF PTC, APPLICATION OF PTCPinner pyrimidine synthesis



Pinner pyrimidine synthesisJACOB THON BIOR
Ėý
This document summarizes the Pinner pyrimidine synthesis reaction. The reaction involves the condensation of a non-N-substituted amidine and Îē-keto ester (Îē-diketone) in the presence of an acid catalyst to form a pyrimidine heterocyclic ring. The mechanism proceeds through protonation, nucleophilic attack, dehydration, and deprotonation steps. Pyrimidine derivatives synthesized via this reaction are used in drugs to treat conditions like malaria, viruses, and cancer.SYNTHON APPROACH



SYNTHON APPROACHShikha Popali
Ėý
THE SYNTHON APPROACH DECRIBED IN THIS PRESENTATION DETAIL ACCOUNT ON SYNETHESIS WITH STRUCTURE, BASIC RULES, APPLICATIONS.ultrasound assisted reaction 2.pptx



ultrasound assisted reaction 2.pptxTahminaKhan20
Ėý
This document discusses types of sonochemical reactions, specifically heterogeneous solid/liquid and liquid/liquid reactions, and their synthetic applications. Heterogeneous solid/liquid reactions involve solids dispersed in liquids, where ultrasound enhances reactivity by cleaning the solid surface and increasing its effective area through cavitation bubble implosions and microstreaming. Heterogeneous liquid/liquid reactions generate emulsions that increase the interfacial contact area between immiscible liquids, allowing reactions to occur more readily across phase boundaries. Several examples of synthetic applications of these sonochemical reactions are provided, including esterification, saponification, Cannizzaro reactions, and Ullmann-type coupling reactions, which see increased yields, lower temperatures, or shorter reactionHeterocyclic compounds



Heterocyclic compoundspriyaswain27
Ėý
The document discusses several heterocyclic compounds including quinolines, isoquinolines, and indoles. It summarizes key reactions used to synthesize these compounds, including the Combes, Friedlander, Knorr, and Skraup reactions for quinoline synthesis. It also discusses the Bischler-Napieralski, Pictet-Spengler, and Pomeranz-Fritsch reactions for isoquinoline synthesis and the Fischer, Madelung, and Reissert reactions for indole synthesis, along with mechanisms and examples of each reaction. Reactivity and substitution patterns are also covered for quinolines, isoquinolines and indoles.Protection for carboxylic group & Protection for the Amino group



Protection for carboxylic group & Protection for the Amino groupCollege of Pharmacy,Sri Ramakrishna Institute of Paramedical Sciences,Coimbatore
Ėý
Similar to Shapiro reaction (20)
Aromatic rearrangements



Aromatic rearrangementsMISHUSINGH1
Ėý
The document discusses several aromatic rearrangement reactions including the Fries rearrangement, photo-Fries rearrangement, Reimer-Tiemann reaction, Gattermann-Koch reaction, Gattermann aldehyde synthesis, and Vilsmeier-Haack reaction. The Fries rearrangement involves the rearrangement of phenolic esters to ortho- and para-substituted phenolic ketones/aldehydes using Lewis or BrÃļnsted acids. The photo-Fries rearrangement is similar but uses light instead of acids. The Reimer-Tiemann, Gattermann-Koch, and Vilsmeier-Haack reactions all involve the formylation of aromatic compounds to produceImportant Name reaction 



Important Name reaction Home
Ėý
Name reactions:Clemmensen reduction, Darzens condensation, Diels Alder, Eschweiler-Clarke, Friedel-Crafts, Grinard, HofmanMannich, Michael, Meerwin- Pondorf- Verley, Perkin, Reformatsky, Reimer-Tiemann,, Witting and Wolf-Kishner reduction, Aldol condensation.
Chichibabin Reaction



Chichibabin ReactionPRUTHVIRAJ K
Ėý
The Chichibabin reaction is a method for producing 2-aminopyridine derivatives by the reaction of pyridine with sodium amide. It was reported by Aleksei Chichibabin in 1914. The following is the overall form of the general reaction: The direct amination of pyridine with sodium amide takes place in liquid ammoniaIsoquinoline.pptx



Isoquinoline.pptxMohammad Rana Hossain
Ėý
Isoquinoline is a heterocyclic aromatic organic compound that is a structural isomer of quinoline. It consists of a benzene ring fused to a pyridine ring. Isoquinoline derivatives have many pharmaceutical applications including use as antispasmodics, antitussives, anesthetics, and in the production of morphine and related alkaloids. Isoquinoline can be prepared through the Bischler-Napieralski synthesis which involves the condensation and rearrangement of 2-phenylethylamine or through the reaction of cinnamaldehyde with hydroxylamine. Isoquinoline undergoes electrophilic aromatic substitution, oxidation, and reduction reactions.named reaction.pptx



named reaction.pptxDhanashreeKavhale
Ėý
Presented by Dhanashree Kavhale. M. Pharm.(Pharmaceutical Chemistry) 1st year.
Various organic named reactions are there in Advanced Organic Chemistry I, as some of them are explained along with their mechanism.Solventless reaction in green chemistry



Solventless reaction in green chemistryAfrin Nirfa
Ėý
Solventless reactions have gained popularity in green chemistry as they avoid using toxic organic solvents. Some key advantages are that they are more efficient and selective than solvent-based reactions. They also reduce costs by saving on solvents, simplify purification without needing to remove solvent, and are more environmentally friendly. Common solventless reactions include halogenation, Michael additions, aldol condensations, and oxidative couplings of phenols. While homogeneous reactants are required and solvents may still be used during workup, solventless reactions provide an important technique for greener organic synthesis.indole-200409095121.pptx



indole-200409095121.pptxJaved Iqbal
Ėý
The document discusses indole, an aromatic heterocyclic compound consisting of a benzene ring fused to a pyrrole ring. It notes that indole and its substituted derivatives have diverse biological activities and are found in natural products and pharmaceuticals. Some key points made in the document include:
- Indole derivatives are used in many drug classes including antihypertensives, antidepressants, antipsychotics, NSAIDs, and more.
- Several total syntheses of complex indole natural products are described, utilizing reactions like intermolecular indole aryne cycloaddition.
- Methods for synthesizing substituted indoles are reviewed, such as the Fischer indole synthesis, Zn(OTf)2100 named reactions



100 named reactionsRoshen Reji Idiculla
Ėý
100 named reactions with examples of total syntheses which utilized these reactions, with reaction conditions. with included references for each syntheses.Condensation reactions 



Condensation reactions Institute of Chemical Technology
Ėý
Condensation reactions involve the combination of two molecules with the loss of a small molecule like water. Aldol condensation forms carbon-carbon bonds by reacting an enolate ion with a carbonyl compound. It plays a role in gluconeogenesis, photosynthesis, and producing perfumes. Other condensation reactions discussed include Claisen, Knoevenagel, Schiff base formation, and Dieckmann cyclization. Condensation polymers are formed through condensation reactions and examples include nylon and DNA. Condensation reactions are widely used in organic synthesis and producing pharmaceuticals, fragrances, and polymers.Organic reaction



Organic reactionSanjay Gopi
Ėý
This document summarizes several organic reactions including the Mannich reaction, ozonolysis, Mitsunobu reaction, and Baeyer-Villiger oxidation. It provides the mechanisms and synthetic applications of each reaction. The Mannich reaction involves the condensation of an active methyl group in a ketone. Ozonolysis is used to cleave carbon-carbon double bonds and locate their position. The Mitsunobu reaction converts alcohols to other functional groups like esters with inversion of stereochemistry. Baeyer-Villiger oxidation uses a peroxycarboxylic acid to convert a ketone to an ester.QUINOLINE, ISOQUINOLINE AND INDOLE



QUINOLINE, ISOQUINOLINE AND INDOLEPharma Rising, Bhopal
Ėý
The document provides information about quinoline, isoquinoline, and indole. It discusses their structures, properties, synthesis methods, and reactions. Quinoline and isoquinoline are both heterocyclic aromatic compounds composed of a benzene ring fused to a pyridine ring. They undergo similar electrophilic and nucleophilic substitution reactions. Common synthesis routes for quinoline include the Skraup, Doebner-Miller, and Conrad-Limpach reactions. Isoquinoline synthesis methods include the Pomeranz-Fritsch and Bischler-Napieralski reactions. Indole is a bicyclic molecule with a benzene and pyrrole ring. It does not readilyChapter-2.pptx



Chapter-2.pptxJaved Iqbal
Ėý
This document discusses several 5-membered heterocyclic compounds including furan, thiophene, and pyrrole. It outlines their general structures, with one carbon atom replaced by an oxygen in furan, sulfur in thiophene, and nitrogen in pyrrole. Several common methods for synthesizing these heterocycles are also presented, such as the Paal-Knorr reaction involving cyclization of 1,4-diketones or analogous compounds. The document concludes by comparing the reactivity of these heterocycles towards electrophilic aromatic substitution and other reaction types.vdocuments.net_heterocyclic-chemistry-58bb82e5b406c.ppt



vdocuments.net_heterocyclic-chemistry-58bb82e5b406c.pptJaved Iqbal
Ėý
This document discusses several 5-membered heterocyclic compounds: furan, thiophene, pyrrole, imidazole, and indole. It outlines common strategies for synthesizing heterocycles, including ring closure methods, the "4+1" and "5+1" strategies, and manipulation of oxidation states. Electrophilic aromatic substitution is discussed as a major reaction type for these compounds, with reactivity generally following the order pyrrole > furan > thiophene > benzene. Oxidation and reduction reactions are also covered.REACTIONS.of organic chemistry pptx (.).



REACTIONS.of organic chemistry pptx (.).imranjan5261
Ėý
This document summarizes several organic reactions covered in an advanced chemistry course. It describes the Baeyer-Villiger oxidation reaction which forms esters from ketones using peroxyacids. It also discusses the Diels-Alder reaction, Wolff-Kishner reduction, Friedel-Crafts reactions, Perkin reaction and Cannizzaro's reaction, providing examples, mechanisms and applications for each.Name-Reaction-2 (1).pptx



Name-Reaction-2 (1).pptxTayari
Ėý
The Reformatsky reaction condenses aldehydes or ketones with Îą-halo esters using zinc metal to form Îē-hydroxy esters. The reaction proceeds via an organozinc reagent or 'Reformatsky enolate' formed by treating the Îą-halo ester with zinc dust. This enolate then reacts with the carbonyl group in a concerted reaction involving a six-membered transition state to form the carbon-carbon bond. Workup with acid removes zinc and provides the Îē-hydroxy ester product. The main application is the preparation of Îē-hydroxy esters from aldehydes or ketones.Metathesis



MetathesisMISHUSINGH1
Ėý
This document discusses metathesis reactions and their applications in organic synthesis. It begins with definitions and examples of different types of metathesis reactions including alkene, alkyne, and enyne metathesis. It then covers the key catalysts used, such as Grubbs and Schrock catalysts, as well as the 2005 Nobel Prize awarded for the development of metathesis reactions. The document concludes by outlining several important applications of metathesis in synthesizing biologically active compounds and natural products.Aromatic electrophilic substitution reactions.pptx



Aromatic electrophilic substitution reactions.pptxTamralipta Mahavidyalaya
Ėý
Electrophilic aromatic substitution reactions are organic reactions wherein an electrophile replaces an atom which is attached to an aromatic ring. Commonly, these reactions involve the replacement of a hydrogen atom belonging to a benzene ring with an electrophileCommon named reactions 



Common named reactions shekhar suman
Ėý
The document discusses several carbon-carbon bond forming reactions:
1. Aldol condensation allows aldehydes and ketones to undergo self-condensation in the presence of a base to form Îē-hydroxy carbonyl compounds.
2. The Perkin reaction uses an acid anhydride to form Îą,Îē-unsaturated aromatic acids from aromatic aldehydes.
3. The Wittig reaction converts a carbonyl group to an alkene using a phosphonium ylide.Vilsmeir reagent reactions by Perumal 



Vilsmeir reagent reactions by Perumal Ashok Kumar
Ėý
This is ppt presentation of Dr. P.T. Perumal on the topic of preparation and reactions various Vilsmeir reagent and their applications in Heterocyclic chemistry.
This is very useful presentation and will be useful as a good reference for work on Heterocyclic chemistry.SBrand_VLA4



SBrand_VLA4Stephen Brand
Ėý
This document summarizes the efficient synthesis of novel 3-aminocyclobut-2-en-1-ones, which are potent antagonists of VLA-4. The synthesis involves the condensation of cyclobuta-1,3-diones with a phenylalanine-derived primary amine. This produces vinylogous amide derivatives in good yields. The cyclobuta-1,3-diones are prepared through cycloaddition of ketene intermediates, generated from acid chlorides, with various alkynes. The resulting 3-aminocyclobut-2-en-1-ones can then be functionalized at C-2 through reaction with electrophilic reagentsRecently uploaded (20)
Azure Administrator Interview Questions By ScholarHat



Azure Administrator Interview Questions By ScholarHatScholarhat
Ėý
Azure Administrator Interview Questions By ScholarHatDot NET Core Interview Questions PDF By ScholarHat



Dot NET Core Interview Questions PDF By ScholarHatScholarhat
Ėý
Dot NET Core Interview Questions PDF By ScholarHatHow to Configure Recurring Revenue in Odoo 17 CRM



How to Configure Recurring Revenue in Odoo 17 CRMCeline George
Ėý
This slide will represent how to configure Recurring revenue. Recurring revenue are the income generated at a particular interval. Typically, the interval can be monthly, yearly, or we can customize the intervals for a product or service based on its subscription or contract. Blind spots in AI and Formulation Science, IFPAC 2025.pdf



Blind spots in AI and Formulation Science, IFPAC 2025.pdfAjaz Hussain
Ėý
The intersection of AI and pharmaceutical formulation science highlights significant blind spotsâsystemic gaps in pharmaceutical development, regulatory oversight, quality assurance, and the ethical use of AIâthat could jeopardize patient safety and undermine public trust. To move forward effectively, we must address these normalized blind spots, which may arise from outdated assumptions, errors, gaps in previous knowledge, and biases in language or regulatory inertia. This is essential to ensure that AI and formulation science are developed as tools for patient-centered and ethical healthcare.Intellectual Honesty & Research Integrity.pptx



Intellectual Honesty & Research Integrity.pptxNidhiSharma495177
Ėý
Research Publication & Ethics contains a chapter on Intellectual Honesty and Research Integrity.
Different case studies of intellectual dishonesty and integrity were discussed.Odoo 18 Accounting Access Rights - Odoo 18 šÝšÝßĢs



Odoo 18 Accounting Access Rights - Odoo 18 šÝšÝßĢsCeline George
Ėý
In this slide, weâll discuss on accounting access rights in odoo 18. To ensure data security and maintain confidentiality, Odoo provides a robust access rights system that allows administrators to control who can access and modify accounting data. Comprehensive Guide to Antibiotics & Beta-Lactam Antibiotics.pptx



Comprehensive Guide to Antibiotics & Beta-Lactam Antibiotics.pptxSamruddhi Khonde
Ėý
ðĒ Comprehensive Guide to Antibiotics & Beta-Lactam Antibiotics
ðŽ Antibiotics have revolutionized medicine, playing a crucial role in combating bacterial infections. Among them, Beta-Lactam antibiotics remain the most widely used class due to their effectiveness against Gram-positive and Gram-negative bacteria. This guide provides a detailed overview of their history, classification, chemical structures, mode of action, resistance mechanisms, SAR, and clinical applications.
ð What Youâll Learn in This Presentation
â
History & Evolution of Antibiotics
â
Cell Wall Structure of Gram-Positive & Gram-Negative Bacteria
â
Beta-Lactam Antibiotics: Classification & Subtypes
â
Penicillins, Cephalosporins, Carbapenems & Monobactams
â
Mode of Action (MOA) & Structure-Activity Relationship (SAR)
â
Beta-Lactamase Inhibitors & Resistance Mechanisms
â
Clinical Applications & Challenges.
ð Why You Should Check This Out?
Essential for pharmacy, medical & life sciences students.
Provides insights into antibiotic resistance & pharmaceutical trends.
Useful for healthcare professionals & researchers in drug discovery.
ð Swipe through & explore the world of antibiotics today!
ð Like, Share & Follow for more in-depth pharma insights!Inventory Reporting in Odoo 17 - Odoo 17 Inventory App



Inventory Reporting in Odoo 17 - Odoo 17 Inventory AppCeline George
Ėý
This slide will helps us to efficiently create detailed reports of different records defined in its modules, both analytical and quantitative, with Odoo 17 ERP.RRB ALP CBT 2 RAC Question Paper MCQ (Railway Assistant Loco Pilot)



RRB ALP CBT 2 RAC Question Paper MCQ (Railway Assistant Loco Pilot)SONU HEETSON
Ėý
RRB ALP CBT 2 RAC Question Paper MCQ PDF Free Download. Railway Assistant Loco Pilot Mechanic Refrigeration and Air Conditioning Important Questions.Year 10 The Senior Phase Session 3 Term 1.pptx



Year 10 The Senior Phase Session 3 Term 1.pptxmansk2
Ėý
Year 10 The Senior Phase Session 3 Term 1.pptxOne Click RFQ Cancellation in Odoo 18 - Odoo šÝšÝßĢs



One Click RFQ Cancellation in Odoo 18 - Odoo šÝšÝßĢsCeline George
Ėý
In this slide, weâll discuss the one click RFQ Cancellation in odoo 18. One-Click RFQ Cancellation in Odoo 18 is a feature that allows users to quickly and easily cancel Request for Quotations (RFQs) with a single click.ASP.NET Web API Interview Questions By Scholarhat



ASP.NET Web API Interview Questions By ScholarhatScholarhat
Ėý
ASP.NET Web API Interview Questions By ScholarhatHow to Configure Proforma Invoice in Odoo 18 Sales



How to Configure Proforma Invoice in Odoo 18 SalesCeline George
Ėý
In this slide, weâll discuss on how to configure proforma invoice in Odoo 18 Sales module. A proforma invoice is a preliminary invoice that serves as a commercial document issued by a seller to a buyer.Functional Muscle Testing of Facial Muscles.pdf



Functional Muscle Testing of Facial Muscles.pdfSamarHosni3
Ėý
Functional Muscle Testing of Facial Muscles.pdfShapiro reaction
- 1. SHAPIRO REACTION [TOSYL HYDRAZONE DECOMPOSITION] SUBMITTED BY : RINSHANA FATHIMA ABDUL AZEEZ FIRST YEAR M.PHARM PHARMACEUTICAL CHEMISTRY AL SHIFA COLLEGE OF PHARMACY 1
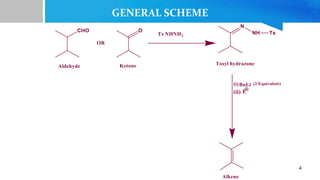
- 2. âĒ Discovered by Robert H. Shapiro in 1967 âĒ Shapiro reaction (1967) involves the conversion of aryl sulfonyl hydrazones of aldehydes and ketones into olefins by reacting with alkyl lithium reagent, grignard reagent, or alkali metal amide.[1] âĒ This reaction is carried out at a temperature of -78â 2
- 3. The reaction similar to Bamford Stevens reaction where bases such as Na, NaOMe, LiH, NaH, NaNH2, etc. are used. Another major difference between the two reactions is that Shapiro reaction yields less substitued olefins as kinetcic products (the product that predominates when the reaction is done at low temperature.) While Bamford Stevens reaction yields more substituted olefins as the thermodynamic product (the product that predominates when the reaction is done at high temperature.)[2] 3
- 5. âĒ Tosyl hydrazone is a functional group with the general structure RR'C=N-NH-Ts , where Ts is a tosyl group. âĒ Tosyl or Toluene sulfonyl group with the molecular formula CH3C6H4SO2 âĒ This is used as âProtecting Groupâ for alcohols and amines. âĒ Proctecting groups are introduced in to a molecule by chemical modification of a functional group to obtain chemoselectivity in a multistep reaction. 5
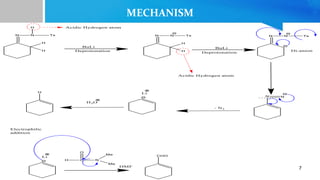
- 6. MECHANISM 1. Deprotonation of -NH proton from aryl sulfonyl hydrazone. 2. Second deprotonation adjacent to the hydrazone group to give a dianion. 3. Elimination of toluene sulfinate to form an intermediate (carbanion mechanism). 4. Loss of N2 to form alkenylithium species which is then protonated (treated with an electrophile) to give olefins.[1] 6
- 7. MECHANISM 7
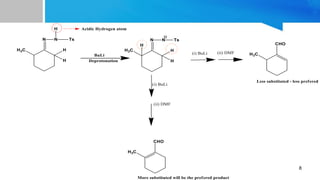
- 8. 8
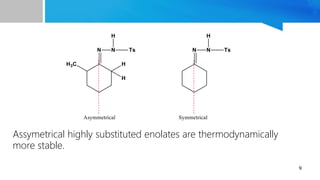
- 9. 9 Assymetrical highly substituted enolates are thermodynamically more stable.
- 10. 10
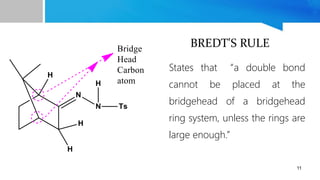
- 11. 11 BREDT'S RULE States that âa double bond cannot be placed at the bridgehead of a bridgehead ring system, unless the rings are large enough.â
- 12. âĒ Synthesis of an allylic alchol, which is an intermediate in the synthesis of Mequitazine, Which is an H1 anti histaminic used to treat allergies and rhinitis. [5] 12 SYNTHETIC APPLICATIONS
- 13. âĒ (-)-phytocassane D was synthesized from (R)-Wieland Miescher Ketone by this reaction, for determining the absolute configuration of the phytocassane group of the phytoalexins.[3] 13 WMK is a racemic bicyclic diketone used in the total synthesis of more than 50 natural compounds
- 14. âĒ Shapiro reaction is involved in the formation of ring B in the Nicolaou Taxol total synthesis.[4] A B D C 14
- 15. âĒ A class of Chiral indenes (Verbindenes) was prepared from Verbenone by utilising Shapiro reaction as one of the key transformation step.[3] 15
- 16. REFERENCE 1. Jie Jack Li; Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications Fifth Edition; Springer Science & Business Media, 30-Jan-2014; Page no. 544 2. Jie Jack Li; Name Reactions Third Edition; Springer Science & Business Media, 2006; Page no.529 3. Laszlo Kurti, Barbara Czako; Strategic Applications of Named Reactions in Organic Synthesis; Elsevier, 29-Apr-2005; Page no. 37 16
- 17. 4. Pei-Qiang Huang, Zhu-Jun Yao, Richard P. Hsung; Efficiency in Natural Product Total Synthesis; John Wiley & Sons, 24-Jul-2018; Page no.9- 93. 5. https://patents.google.com/patent/US20100105897 17 REFERENCE
- 18. THANK YOU 18





