Solid State Class 12 Chemistry RBSE or CBSE
Download as PPTX, PDF10 likes1,245 views
Solid state always confuse and I have tried easy way so that students can learn this chapter very easily.
1 of 12
Downloaded 74 times



Recommended
Solid state



Solid stateRamakrishna Ager
╠²
Solids can be either crystalline or amorphous. Crystalline solids have a definite shape and sharp melting point, while amorphous solids lack a definite shape and melting point. There are two main types of crystalline solids: ionic crystals composed of ions and molecular crystals composed of molecules. Ionic crystals form lattice structures with ions arranged in repeating patterns. The characteristics and structures of different types of ionic crystals depend on factors like the ions' sizes and their ratio. Solid state 2017



Solid state 2017nysa tutorial
╠²
this is based on jee, nee, aipmt, maharahstra board, icse and cbse board. if you want this ppt to download please donate me Rs200/- for my each hard dedicated ppt. put on my paytm acount- 8879919898.
you can also go to my site-
www.akchem.blogspot.com
contact - aktr000@gmail.comIonic solids



Ionic solidsakshay kulshrestha
╠²
Ionic solids are composed of positively charged cations and negatively charged anions arranged in a 3D array. The electrostatic attractions between opposite charges hold the ions in fixed positions, making ionic solids hard and brittle. The melting point of ionic solids is generally over 150 degrees C because strong electrostatic forces must be overcome for melting to occur. The radius ratio rule can be used to predict the coordination number of ions based on the ratio of cation to anion radii, with different ratios corresponding to different coordination geometries like tetrahedral or octahedral. While useful, the radius ratio rule has limitations as it treats ions as hard spheres and does not account for variations in effective ionic radii.Crystalline Solids



Crystalline SolidsSidra Javed
╠²
This document discusses different types of crystalline solids. It defines a crystalline solid as having a well-ordered structure with definite arrangements of particles. Crystalline solids are made up of repeating units called unit cells, which together form a crystal lattice. The document describes the different packing arrangements of particles in unit cells and classifies crystalline solids into four main types - ionic, covalent, metallic and molecular crystals - based on the type of bonding forces between particles. Each type of crystalline solid is characterized by distinct properties like melting point, conductivity, hardness and thermal stability.Solid



SolidPoonam Singh
╠²
The document summarizes key concepts about crystalline and amorphous solids, including:
- Crystalline solids exhibit long-range order while amorphous solids only have short-range order.
- Ionic crystals like NaCl and CsCl form face-centered cubic or body-centered cubic structures to maximize interactions between oppositely charged ions.
- The cohesive energy of ionic crystals can be calculated by considering contributions from Coulomb attraction, electron overlap repulsion, ionization energies, and electron affinities.solid state



solid statemadan kumar
╠²
This document provides an overview of solid state physics. It discusses the main types of solids as crystalline and amorphous. Crystalline solids have a long-range ordered structure while amorphous solids lack long-range order. It also describes different crystal structures like unit cells, Bragg's equation for determining crystal structure from X-ray diffraction, and the four main types of crystals: molecular, covalent, metallic and ionic.Solid state chemistry ppt



Solid state chemistry pptAkhtarShah8
╠²
This document provides an introduction to solid state properties including crystalline and amorphous solids. It defines key solid state concepts such as polymorphism, allotrophism, isotropic and anisotropic substances. It also describes techniques for analyzing crystal structure including X-ray diffraction, Bragg's equation, unit cells, Miller indices and powder methods. Semiconductors are discussed as materials that can conduct electricity depending on temperature and impurities. Solar cells are mentioned as an application that uses a p-n junction formed from a p-type and n-type semiconductor.Characteristics of crystalline solid



Characteristics of crystalline solidSagar Dutta
╠²
This document provides an overview of solid state chemistry and properties of solid surfaces. It discusses the following key points:
- Solids have definite shapes and volumes due to strong forces holding their atoms, molecules, or ions in fixed positions. This gives solids their rigidity and mechanical strength.
- There are two main types of solids - crystalline solids which have a regular repeating structure and amorphous solids which lack long-range order.
- Techniques for characterizing solid surfaces include low-energy electron diffraction (LEED) and X-ray photoelectron spectroscopy (XPS) which can provide information about surface structure and composition.
- LEED specifically works by bombarding a crystalline surfaceSolid state class 12 CBSE



Solid state class 12 CBSEritik
╠²
The document discusses the characteristics of solids and different types of crystalline structures. It describes that solids can be crystalline or amorphous based on the ordering of particles. Crystalline solids have long-range order and a repeating pattern, while amorphous solids only have short-range order. Crystalline solids are further classified as ionic, molecular, metallic or covalent networks based on bonding. Crystals consist of lattice structures with primitive or centered unit cells containing particles in specific arrangements. Close packing of spheres in one, two or three dimensions results in different crystal structures like simple cubic, body centered cubic or hexagonal close packed.Solids - Arrangement of solid particles



Solids - Arrangement of solid particlesSidra Javed
╠²
In solids, molecules, ions or atoms are arranged in a definite pattern. Packing arrangement of particles is responsible for different types of solids and their properties Types of solids



Types of solidsMaramandansubu
╠²
There are two main types of solids: crystalline and amorphous. Crystalline solids have a regular, ordered atomic arrangement forming a crystal lattice, while amorphous solids lack long-range order and have an irregular, haphazard structure. Examples of crystalline solids include NaCl and CsCl, while examples of amorphous solids include glass and rubber. Crystalline solids can also be classified based on their crystal structure as ionic, covalent, molecular, or metallic.CHEMICAL ENGG. MATERIALS- CRYSTALLINE & NON-CRYSTALLINE SOLIDS



CHEMICAL ENGG. MATERIALS- CRYSTALLINE & NON-CRYSTALLINE SOLIDSAjinkya Khandizod
╠²
Difference between CRYSTALLINE & NON-CRYSTALLINE SOLIDS , useful for studying subject like CHEMICAL ENGG. MATERIALSStructure of solids



Structure of solidsProf. A.Balasubramanian
╠²
Solids are characterized by their definite shape and also their considerable mechanical strength and rigidity. The particles that compose a solid material(with few exceptions), whether ionic, molecular, covalent or metallic, are held in place by strong attractive forces between them.Classification of crystalline solids



Classification of crystalline solidsManoj Er
╠²
Crystalline solids can be classified into four main categories based on the type of intermolecular forces present: molecular solids, ionic solids, metallic solids, and covalent solids. Molecular solids are further divided into nonpolar, polar, and hydrogen-bonded types. Ionic solids are formed from electrostatic attractions between cations and anions. Metallic solids contain a sea of free electrons that allow for high conductivity. Covalent solids form giant covalent structures with strong directional bonds, making them very hard and with high melting points.Solid state 



Solid state Vishwas Agarwal
╠²
This document provides an overview of solid state structures. It discusses the two main types of solids - crystalline and amorphous - and explains their distinguishing characteristics. Crystalline solids have a definite, orderly arrangement of atoms while amorphous solids do not. The document then covers various topics related to crystalline solids, including crystal structures, unit cells, Bravais lattices, and the structures of materials like NaCl, diamond, and graphite. It also discusses crystal imperfections and different types of defects that can occur in ionic crystals.solid state



solid statenandang mufti
╠²
The document discusses solid state physics and the properties of solid materials. It explains that solid state physics formulates laws governing the behavior of solids and explores why materials like carbon can exist in different states with varying electrical properties. The document also classifies solids as crystalline, polycrystalline, or amorphous and discusses crystal structure, lattice, and unit cells - the basic repeating units that make up crystalline solids. Understanding these atomic arrangements is important for explaining the behavior and properties of different materials.Module2



Module2rpclemson
╠²
This document discusses the atomic arrangement and properties of crystalline solids such as metals. It begins by describing the long-range order in crystalline solids compared to the short-range order in amorphous solids. It then discusses various crystal structures including cubic, hexagonal, and body-centered cubic. It provides examples of calculating properties like atomic packing factor and theoretical density based on crystal structure. Finally, it discusses using X-ray diffraction to determine crystal structure by measuring spacing between crystal planes.Classification of crystalline solids



Classification of crystalline solidsMaramandansubu
╠²
This document describes four main types of crystalline solids: ionic solids, which are hard and brittle with strong electrostatic forces between positive and negative ions; covalent solids, which are hard with strong covalent bonds between neutral atoms like diamond and graphite; molecular solids, which are soft with weak van der Waals forces between neutral molecules; and metallic solids, which have mobile electrons conducting electricity and positive metal ions giving properties of malleability, ductility, and high tensile strength.The solid state



The solid stateMukul Kumar
╠²
This document summarizes different types of solids. Crystalline solids have long-range order between particles which gives them distinct properties like sharp melting points and anisotropy. Amorphous solids only have short-range order, making them similar to supercooled liquids with no distinct melting point. Crystalline solids can be classified as molecular, ionic, metallic or covalent based on the interactions between particles. Each type of solid has distinct physical properties depending on these interactions.SOLID STATE -XII BY SULEKHA RANI R , PGT CHEMISTRY



SOLID STATE -XII BY SULEKHA RANI R , PGT CHEMISTRYSulekha Nisanth
╠²
Here are the definitions and differences you asked for:
Short range order - Atoms are arranged in a disordered manner within a small region but this arrangement does not extend over long distances.
Anisotropic - A material whose physical properties vary with the direction of measurement.
Unit cell - The smallest repeating unit that constructs the entire crystal by translation.
Voids - Empty spaces between closely packed spheres in crystal structures. There are two types - octahedral and tetrahedral voids.
Impurity defect - Occurs when an atom of one element replaces an atom of the host element in its normal lattice position.
Monoclinic - Unit cell with two axes at 90 degrees and one axis not at 90 degreesCrystalline solids !



Crystalline solids !Usman Shah
╠²
I hope You all like it. I hope It is very beneficial for you all. I really thought that you all get enough knowledge from this presentation. This presentation is about materials and their classifications. After you read this presentation you knowledge is not as before.Solid state chemistry



Solid state chemistryKumar
╠²
This document discusses solid state chemistry and provides information on various topics within the subject. It begins by defining the three states of matter and what distinguishes a solid. It then describes the two main types of solids - crystalline and amorphous - and provides details on their structures and properties. Various types of crystal structures are also outlined, including ionic, covalent, molecular and metallic crystals. The document concludes by discussing Bragg's equation and important solid materials like diamond, graphite and fullerenes.Crystal structure of metals and semiconductors

Crystal structure of metals and semiconductorsMUKHTIAR HUSSAIN
╠²
The document discusses the crystal structures of materials. It begins by explaining that the properties of some materials are directly related to their crystal structures. For example, magnesium and beryllium have different properties than gold and silver due to differences in their crystal structures. It then lists the key learning objectives which include describing different crystal structures, computing densities, and distinguishing between single crystals and polycrystalline materials. The document goes on to explain common metallic crystal structures like body centered cubic and face centered cubic, as well as non-metallic structures like rock salt and cesium chloride. It also discusses factors that determine crystal structure such as the relative sizes of ions to maximize interactions and maintain charge neutrality.Crystalline solids (group 1)



Crystalline solids (group 1)S M
╠²
There are three main types of crystalline solids: ionic solids, molecular solids, and metallic solids. Ionic solids are composed of positive and negative ions arranged in a crystal lattice. They have properties like high melting points and are brittle. Molecular solids have molecules arranged in a particular configuration, and properties like low melting points and being nonconductors. Metallic solids have metal atoms or ions arranged in patterns, giving properties such as conductivity and malleability. All crystalline solids have constituents ordered in highly organized, repeating microscopic structures extending in three dimensions.Gseb class 12 chemistry sem 3 ch 1 solid state part 1



Gseb class 12 chemistry sem 3 ch 1 solid state part 1Saumil Sharma
╠²
There are five states of matter: solid, liquid, gas, plasma and Bose-Einstein condensate. The kinetic molecular theory explains the differences between these states. Solids have a definite shape and arrangement of particles, which can only vibrate in fixed positions. Solids are classified based on their molecular structure and bonding forces between particles as crystalline or amorphous, and further as molecular, ionic, metallic or covalent. Crystalline solids have long-range orderly arrangements while amorphous solids only have short-range order.Chemistry chapter wise important questions



Chemistry chapter wise important questionsSrikanth KS
╠²
Chapter wise important questions in Chemistry for Karnataka 2 year PU Science students. This is taken from the PU board website and compiled together.Solid state physics - Crystalline Solids



Solid state physics - Crystalline SolidsAthren Tidalgo
╠²
This document discusses condensed matter systems and crystalline solids. It describes hard matter as including crystalline solids which can be conductors, semiconductors or insulators, as well as crystalline solids with defects. Soft matter includes colloidal dispersions, polymer melts and solutions, liquid crystals and biomatter. Conductors allow the flow of charges across their surface. Insulators resist current flow. Semiconductors have conductivity between conductors and insulators and can control electron flow depending on applied energy. Band theory and band gaps influence the electrical and optical properties of materials.Lecture 03



Lecture 03luyenkimnet
╠²
This document discusses defects in ceramics and how to determine crystallographic directions and planes. It defines common ionic defects like vacancies, interstitials, and impurities that can make a ceramic non-stoichiometric. Defects must maintain charge neutrality. Directions and planes are identified using Miller indices and their families can be drawn for common structures like NaCl and ZnS. Dopants are purposefully added to modify properties while impurities cannot be fully removed.(2096)lecture_notes_solid_state_e.pdf



(2096)lecture_notes_solid_state_e.pdfunnatisinari2
╠²
The document provides the lecture plan for a solid state chemistry course consisting of 7 lectures. The first lecture will cover basics of solid state including definitions of different types of solids and their properties. Lectures 2-4 will cover simple cubic structure, body centered cubic, closest packing, hexagonal close packing, and cubic close packing structures. Lecture 5 will discuss radius ratio rule and types of ionic structures. Lecture 6 will cover crystal defects and properties of solids. The last lecture will complete the solid state course with discussion.Solid state.pdf



Solid state.pdfSUCCESSSCIENCEACADEM
╠²
The document discusses various types of solids and their properties. It describes crystalline solids as having long-range order of particles in a repeating pattern, with examples including NaCl and quartz. Amorphous solids lack long-range order and have only short-range order, appearing solid-like but with non-definite shapes. Properties discussed include rigidity, compressibility, density, and melting behaviors. Crystalline solids are classified further based on their crystal structure and symmetry.More Related Content
What's hot (20)
Solid state class 12 CBSE



Solid state class 12 CBSEritik
╠²
The document discusses the characteristics of solids and different types of crystalline structures. It describes that solids can be crystalline or amorphous based on the ordering of particles. Crystalline solids have long-range order and a repeating pattern, while amorphous solids only have short-range order. Crystalline solids are further classified as ionic, molecular, metallic or covalent networks based on bonding. Crystals consist of lattice structures with primitive or centered unit cells containing particles in specific arrangements. Close packing of spheres in one, two or three dimensions results in different crystal structures like simple cubic, body centered cubic or hexagonal close packed.Solids - Arrangement of solid particles



Solids - Arrangement of solid particlesSidra Javed
╠²
In solids, molecules, ions or atoms are arranged in a definite pattern. Packing arrangement of particles is responsible for different types of solids and their properties Types of solids



Types of solidsMaramandansubu
╠²
There are two main types of solids: crystalline and amorphous. Crystalline solids have a regular, ordered atomic arrangement forming a crystal lattice, while amorphous solids lack long-range order and have an irregular, haphazard structure. Examples of crystalline solids include NaCl and CsCl, while examples of amorphous solids include glass and rubber. Crystalline solids can also be classified based on their crystal structure as ionic, covalent, molecular, or metallic.CHEMICAL ENGG. MATERIALS- CRYSTALLINE & NON-CRYSTALLINE SOLIDS



CHEMICAL ENGG. MATERIALS- CRYSTALLINE & NON-CRYSTALLINE SOLIDSAjinkya Khandizod
╠²
Difference between CRYSTALLINE & NON-CRYSTALLINE SOLIDS , useful for studying subject like CHEMICAL ENGG. MATERIALSStructure of solids



Structure of solidsProf. A.Balasubramanian
╠²
Solids are characterized by their definite shape and also their considerable mechanical strength and rigidity. The particles that compose a solid material(with few exceptions), whether ionic, molecular, covalent or metallic, are held in place by strong attractive forces between them.Classification of crystalline solids



Classification of crystalline solidsManoj Er
╠²
Crystalline solids can be classified into four main categories based on the type of intermolecular forces present: molecular solids, ionic solids, metallic solids, and covalent solids. Molecular solids are further divided into nonpolar, polar, and hydrogen-bonded types. Ionic solids are formed from electrostatic attractions between cations and anions. Metallic solids contain a sea of free electrons that allow for high conductivity. Covalent solids form giant covalent structures with strong directional bonds, making them very hard and with high melting points.Solid state 



Solid state Vishwas Agarwal
╠²
This document provides an overview of solid state structures. It discusses the two main types of solids - crystalline and amorphous - and explains their distinguishing characteristics. Crystalline solids have a definite, orderly arrangement of atoms while amorphous solids do not. The document then covers various topics related to crystalline solids, including crystal structures, unit cells, Bravais lattices, and the structures of materials like NaCl, diamond, and graphite. It also discusses crystal imperfections and different types of defects that can occur in ionic crystals.solid state



solid statenandang mufti
╠²
The document discusses solid state physics and the properties of solid materials. It explains that solid state physics formulates laws governing the behavior of solids and explores why materials like carbon can exist in different states with varying electrical properties. The document also classifies solids as crystalline, polycrystalline, or amorphous and discusses crystal structure, lattice, and unit cells - the basic repeating units that make up crystalline solids. Understanding these atomic arrangements is important for explaining the behavior and properties of different materials.Module2



Module2rpclemson
╠²
This document discusses the atomic arrangement and properties of crystalline solids such as metals. It begins by describing the long-range order in crystalline solids compared to the short-range order in amorphous solids. It then discusses various crystal structures including cubic, hexagonal, and body-centered cubic. It provides examples of calculating properties like atomic packing factor and theoretical density based on crystal structure. Finally, it discusses using X-ray diffraction to determine crystal structure by measuring spacing between crystal planes.Classification of crystalline solids



Classification of crystalline solidsMaramandansubu
╠²
This document describes four main types of crystalline solids: ionic solids, which are hard and brittle with strong electrostatic forces between positive and negative ions; covalent solids, which are hard with strong covalent bonds between neutral atoms like diamond and graphite; molecular solids, which are soft with weak van der Waals forces between neutral molecules; and metallic solids, which have mobile electrons conducting electricity and positive metal ions giving properties of malleability, ductility, and high tensile strength.The solid state



The solid stateMukul Kumar
╠²
This document summarizes different types of solids. Crystalline solids have long-range order between particles which gives them distinct properties like sharp melting points and anisotropy. Amorphous solids only have short-range order, making them similar to supercooled liquids with no distinct melting point. Crystalline solids can be classified as molecular, ionic, metallic or covalent based on the interactions between particles. Each type of solid has distinct physical properties depending on these interactions.SOLID STATE -XII BY SULEKHA RANI R , PGT CHEMISTRY



SOLID STATE -XII BY SULEKHA RANI R , PGT CHEMISTRYSulekha Nisanth
╠²
Here are the definitions and differences you asked for:
Short range order - Atoms are arranged in a disordered manner within a small region but this arrangement does not extend over long distances.
Anisotropic - A material whose physical properties vary with the direction of measurement.
Unit cell - The smallest repeating unit that constructs the entire crystal by translation.
Voids - Empty spaces between closely packed spheres in crystal structures. There are two types - octahedral and tetrahedral voids.
Impurity defect - Occurs when an atom of one element replaces an atom of the host element in its normal lattice position.
Monoclinic - Unit cell with two axes at 90 degrees and one axis not at 90 degreesCrystalline solids !



Crystalline solids !Usman Shah
╠²
I hope You all like it. I hope It is very beneficial for you all. I really thought that you all get enough knowledge from this presentation. This presentation is about materials and their classifications. After you read this presentation you knowledge is not as before.Solid state chemistry



Solid state chemistryKumar
╠²
This document discusses solid state chemistry and provides information on various topics within the subject. It begins by defining the three states of matter and what distinguishes a solid. It then describes the two main types of solids - crystalline and amorphous - and provides details on their structures and properties. Various types of crystal structures are also outlined, including ionic, covalent, molecular and metallic crystals. The document concludes by discussing Bragg's equation and important solid materials like diamond, graphite and fullerenes.Crystal structure of metals and semiconductors

Crystal structure of metals and semiconductorsMUKHTIAR HUSSAIN
╠²
The document discusses the crystal structures of materials. It begins by explaining that the properties of some materials are directly related to their crystal structures. For example, magnesium and beryllium have different properties than gold and silver due to differences in their crystal structures. It then lists the key learning objectives which include describing different crystal structures, computing densities, and distinguishing between single crystals and polycrystalline materials. The document goes on to explain common metallic crystal structures like body centered cubic and face centered cubic, as well as non-metallic structures like rock salt and cesium chloride. It also discusses factors that determine crystal structure such as the relative sizes of ions to maximize interactions and maintain charge neutrality.Crystalline solids (group 1)



Crystalline solids (group 1)S M
╠²
There are three main types of crystalline solids: ionic solids, molecular solids, and metallic solids. Ionic solids are composed of positive and negative ions arranged in a crystal lattice. They have properties like high melting points and are brittle. Molecular solids have molecules arranged in a particular configuration, and properties like low melting points and being nonconductors. Metallic solids have metal atoms or ions arranged in patterns, giving properties such as conductivity and malleability. All crystalline solids have constituents ordered in highly organized, repeating microscopic structures extending in three dimensions.Gseb class 12 chemistry sem 3 ch 1 solid state part 1



Gseb class 12 chemistry sem 3 ch 1 solid state part 1Saumil Sharma
╠²
There are five states of matter: solid, liquid, gas, plasma and Bose-Einstein condensate. The kinetic molecular theory explains the differences between these states. Solids have a definite shape and arrangement of particles, which can only vibrate in fixed positions. Solids are classified based on their molecular structure and bonding forces between particles as crystalline or amorphous, and further as molecular, ionic, metallic or covalent. Crystalline solids have long-range orderly arrangements while amorphous solids only have short-range order.Chemistry chapter wise important questions



Chemistry chapter wise important questionsSrikanth KS
╠²
Chapter wise important questions in Chemistry for Karnataka 2 year PU Science students. This is taken from the PU board website and compiled together.Solid state physics - Crystalline Solids



Solid state physics - Crystalline SolidsAthren Tidalgo
╠²
This document discusses condensed matter systems and crystalline solids. It describes hard matter as including crystalline solids which can be conductors, semiconductors or insulators, as well as crystalline solids with defects. Soft matter includes colloidal dispersions, polymer melts and solutions, liquid crystals and biomatter. Conductors allow the flow of charges across their surface. Insulators resist current flow. Semiconductors have conductivity between conductors and insulators and can control electron flow depending on applied energy. Band theory and band gaps influence the electrical and optical properties of materials.Lecture 03



Lecture 03luyenkimnet
╠²
This document discusses defects in ceramics and how to determine crystallographic directions and planes. It defines common ionic defects like vacancies, interstitials, and impurities that can make a ceramic non-stoichiometric. Defects must maintain charge neutrality. Directions and planes are identified using Miller indices and their families can be drawn for common structures like NaCl and ZnS. Dopants are purposefully added to modify properties while impurities cannot be fully removed.Similar to Solid State Class 12 Chemistry RBSE or CBSE (20)
(2096)lecture_notes_solid_state_e.pdf



(2096)lecture_notes_solid_state_e.pdfunnatisinari2
╠²
The document provides the lecture plan for a solid state chemistry course consisting of 7 lectures. The first lecture will cover basics of solid state including definitions of different types of solids and their properties. Lectures 2-4 will cover simple cubic structure, body centered cubic, closest packing, hexagonal close packing, and cubic close packing structures. Lecture 5 will discuss radius ratio rule and types of ionic structures. Lecture 6 will cover crystal defects and properties of solids. The last lecture will complete the solid state course with discussion.Solid state.pdf



Solid state.pdfSUCCESSSCIENCEACADEM
╠²
The document discusses various types of solids and their properties. It describes crystalline solids as having long-range order of particles in a repeating pattern, with examples including NaCl and quartz. Amorphous solids lack long-range order and have only short-range order, appearing solid-like but with non-definite shapes. Properties discussed include rigidity, compressibility, density, and melting behaviors. Crystalline solids are classified further based on their crystal structure and symmetry.Corrosion



CorrosionKANISHKA JHA
╠²
The document discusses corrosion and oxidation of metals, including the principles behind corrosion and methods for preventing corrosion. It describes how metals oxidize when exposed to oxygen and how the formation and properties of metal oxides, such as their adherence and volume, influence corrosion rates. Factors that affect oxidation rates and the development of protective oxide layers are covered. Methods for improving corrosion resistance through alloying and the use of coatings or inhibitors are also summarized.Why Gemstones have multiple Colors___Introduction of Crystal Field Theory & M...



Why Gemstones have multiple Colors___Introduction of Crystal Field Theory & M...Heman Chen
╠²
Gemstones aren't just pretty; they're a science lesson! Colors in gems come from how transition metal ions play with light. Think of it like a dance: ions and ligands groove in a crystal field, splitting energy levels and creating a colorful show. The separation energy, or Ō¢│, is the DJ that controls the party's vibe. Different fields, like square or octahedral, have their own unique beats.
Molecular Orbital Theory adds another layer, with charge transfers and covalent bonds creating color magic. It's like a power duo in a band, where one member (the metal) passes the mic (electrons) to another (the ligand), and voil├Ā, a new color is born.
Energy Band Theory talks about how electrons move in a solid, forming bands that determine if a material conducts or not. It's like a traffic system for electrons, with band gaps being the toll booths.
Physical optical effects, like dispersion and interference, add the final touches to a gem's color palette. It's the gem's structure and properties that paint the picture.Materi 2 bahan kontruksi dan korosi



Materi 2 bahan kontruksi dan korosiDesi Ratna
╠²
The document discusses various types of defects that can occur in crystalline materials, including point defects like vacancies and self-interstitials, and linear defects like dislocations. It explains that all crystals contain vacancies which increase with temperature according to an exponential relationship. Dislocations are line defects characterized by their Burgers vector, and can be either edge, screw, or mixed configurations. Interfacial defects like grain boundaries separate regions with different crystal orientations. These defects influence many material properties.D BLOCK ELEMENTS.pdf389.pdf



D BLOCK ELEMENTS.pdf389.pdfLuckyJoshi9
╠²
D-block elements are those elements belonging to groups 3 through 12 that have their last electron entering the d subshell. Transition elements are defined as elements that have partially filled d orbitals. While all transition elements are d-block elements, not all d-block elements are transition elements as some like zinc have a filled d10 configuration. D-block elements form complex compounds by binding metal ions to anions or neutral molecules through available d orbitals. They also commonly show paramagnetism and catalytic properties due to unpaired electrons in their d orbitals.Chemical effects of electric current



Chemical effects of electric currentDeep Sharma
╠²
Some important short note regarding chemical effects of electric current.
Corrosion



CorrosionManasa Nimmala
╠²
This document discusses corrosion and oxidation of metals. It explains that metals oxidize when exposed to oxygen and the environment. The stability and protective properties of metal oxides depend on factors like their free energy of formation, adherence to the metal surface, and resistance to diffusion. Metals can be protected from corrosion by forming stable oxide layers, alloying with elements like chromium, or using coatings or cathodic protection. Galvanic corrosion can occur when dissimilar metals contact in an electrolyte.┘ģžŁž¦žČž▒ž¦ž¬┘ā┘Ŗ┘ģ┘Ŗž¦žĪ-ž¬┘垦ž│┘é┘Ŗž®-ž¦┘ä┘ģž▒žŁ┘äž®-ž¦┘äž½ž¦┘äž½ž®-┘üžĄ┘ä-ž¦┘ł┘ä.▒Ķ▒Ķ│┘│µ



┘ģžŁž¦žČž▒ž¦ž¬┘ā┘Ŗ┘ģ┘Ŗž¦žĪ-ž¬┘垦ž│┘é┘Ŗž®-ž¦┘ä┘ģž▒žŁ┘äž®-ž¦┘äž½ž¦┘äž½ž®-┘üžĄ┘ä-ž¦┘ł┘ä.▒Ķ▒Ķ│┘│µRiandyPutra1
╠²
This document provides an overview of transition metal coordination chemistry. It discusses the following key points in 3 sentences or less:
Metal complexes consist of a central metal ion bonded to surrounding ligand molecules or ions. The ligands donate lone pairs of electrons to form coordinate covalent bonds with the metal. The geometry and electronic structure of complexes is influenced by the coordination number, ligands present, and hybridization state of the metal ion.chemicalpropertiesofmetals-210506153343 (1).pdf



chemicalpropertiesofmetals-210506153343 (1).pdfkenishavaswani1010
╠²
1) Metals react with oxygen to form metal oxides, with reactivity varying between metals. The most reactive metals, such as sodium and potassium, burn vigorously while copper is the least reactive.
2) Metals also react with water and acids, producing hydrogen gas and alkaline or salt solutions. More reactive metals like sodium and potassium react violently with water.
3) Displacement reactions occur when a more reactive metal is placed in a solution of a less reactive metal salt, displacing the metal from its salt.Chemical Properties of Metals (Class 10)



Chemical Properties of Metals (Class 10)Dr. Pranabjyoti Das
╠²
1) Metals react with oxygen to form metal oxides, with reactivity varying between metals. The most reactive metals, such as sodium and potassium, burn vigorously while copper is the least reactive.
2) Metals also react with water and acids, producing hydrogen gas and salt solutions. More reactive metals like sodium and potassium react violently with water, while less reactive metals do not react or react slowly.
3) When metals react with non-metals, they form ionic compounds through transfer of electrons from the metal to the non-metal. Ionic compounds have high melting points, are brittle solids, and dissolve in water but not organic solvents.4. Bonding (Ionic Bonding)



4. Bonding (Ionic Bonding)Alan Crooks
╠²
The document discusses the nature of chemical bonds and different types of bonding:
1) Ionic bonding occurs between a metal and non-metal atom and involves the transfer of electrons. For example, in sodium chloride, the sodium atom loses an electron to become positively charged and the chlorine atom gains an electron to become negatively charged.
2) Covalent bonding and metallic bonding are the other two main types of chemical bonding at the atomic level.
3) Ionic bonds are very strong electrostatic attractions between oppositely charged ions that form an ionic lattice. This gives ionic substances high melting and boiling points and the ability to conduct electricity when molten or dissolved.Solid_state_pdf.pptx



Solid_state_pdf.pptxDiscoverycoachingIns
╠²
This document discusses different types of solids and their properties. It defines crystalline and amorphous solids, and describes various crystalline solids including molecular, ionic, metallic and covalent solids. It discusses crystal lattices and unit cells, close packing of spheres, and different types of defects that can occur in solids such as stoichiometric defects, non-stoichiometric defects and impurity defects.UNIT-7 s and p Block & Transition Elements.pptx



UNIT-7 s and p Block & Transition Elements.pptxAbdulHannan819750
╠²
This topic is related to inorganic chemistry UN1001_Galvanic Corrosion.ppt



UN1001_Galvanic Corrosion.pptUdyaDevaraja2
╠²
Galvanic corrosion occurs when two dissimilar metals are electrically connected in an electrolyte. The more active metal corrodes preferentially as the anode. Three ways to minimize galvanic corrosion are to select metals close together in the galvanic series, insulate contact between dissimilar metals, and apply protective coatings to the more noble metal.d-block elements



d-block elementsSidra Javed
╠²
d-block elements are those in which the valence electrons enters the d orbital. d- block elements are also called transition elements. Transition elements have partially filled d orbitals.CBSE Class 12 Chemistry Chapter 1 (The Solid State) | Homi Institute



CBSE Class 12 Chemistry Chapter 1 (The Solid State) | Homi InstituteHomi Institute
╠²
The document discusses various types of bonding and intermolecular forces found in solid state materials, including covalent bonding, metallic bonding, ionic bonding, hydrogen bonding, and van der Waals forces. It also describes different types of crystal defects such as point defects, line defects, stoichiometric defects, impurity defects, and non-stoichiometric defects. Finally, it covers topics like semiconductors, ferromagnetism, and the magnetic properties associated with orbiting and spinning electrons.CERAMICS ( as per MGU syllabus)



CERAMICS ( as per MGU syllabus)Denny John
╠²
Ceramic Structures and properties: - coordination number and radius rations - AX,
AmXp, AmBmXp type crystal structures ŌĆō imperfections in ceramics- phase diagrams of
Al2O3 ŌĆō Cr2O3 and MgO- Al2O3 only ŌĆō mechanical properties ŌĆō mechanisms of plastic
deformation ŌĆō ceramic application in heat engine, ceramic armor and electronic
packaging.S block elements (Alkali metals)



S block elements (Alkali metals)Mohamed Minas
╠²
The document discusses the s-block elements, specifically focusing on the alkali metals. It provides an introduction and table of contents. It then discusses the electronic configuration of s-block elements and lists the alkali metals and alkaline earth metals. The next sections provide details on the characteristics properties of alkali metals, including their electronic configuration, atomic and ionic radii, ionization enthalpy, and flame coloration. Further sections describe the atomic and physical properties and chemical properties of alkali metals, including their reactivity towards air, water, hydrogen, and halogens. Applications of some alkali metals are also mentioned. References are listed at the end.giant covalent structures.ppt



giant covalent structures.pptJinsyAjish2
╠²
This document provides an overview of a PowerPoint presentation on structure and bonding for GCSE chemistry students. It introduces ionic bonding, metallic bonding, and covalent bonding. The document explains that the presentation covers how different types of chemical bonding affect the physical properties of elements and compounds. It also provides website information for additional resources on this topic.┘ģžŁž¦žČž▒ž¦ž¬┘ā┘Ŗ┘ģ┘Ŗž¦žĪ-ž¬┘垦ž│┘é┘Ŗž®-ž¦┘ä┘ģž▒žŁ┘äž®-ž¦┘äž½ž¦┘äž½ž®-┘üžĄ┘ä-ž¦┘ł┘ä.▒Ķ▒Ķ│┘│µ



┘ģžŁž¦žČž▒ž¦ž¬┘ā┘Ŗ┘ģ┘Ŗž¦žĪ-ž¬┘垦ž│┘é┘Ŗž®-ž¦┘ä┘ģž▒žŁ┘äž®-ž¦┘äž½ž¦┘äž½ž®-┘üžĄ┘ä-ž¦┘ł┘ä.▒Ķ▒Ķ│┘│µRiandyPutra1
╠²
Recently uploaded (20)
Useful environment methods in Odoo 18 - Odoo ║▌║▌▀Żs



Useful environment methods in Odoo 18 - Odoo ║▌║▌▀ŻsCeline George
╠²
In this slide weŌĆÖll discuss on the useful environment methods in Odoo 18. In Odoo 18, environment methods play a crucial role in simplifying model interactions and enhancing data processing within the ORM framework.Reordering Rules in Odoo 17 Inventory - Odoo ║▌║▌▀Żs



Reordering Rules in Odoo 17 Inventory - Odoo ║▌║▌▀ŻsCeline George
╠²
In Odoo 17, the Inventory module allows us to set up reordering rules to ensure that our stock levels are maintained, preventing stockouts. Let's explore how this feature works.Digital Tools with AI for e-Content Development.pptx



Digital Tools with AI for e-Content Development.pptxDr. Sarita Anand
╠²
This ppt is useful for not only for B.Ed., M.Ed., M.A. (Education) or any other PG level students or Ph.D. scholars but also for the school, college and university teachers who are interested to prepare an e-content with AI for their students and others.How to Setup WhatsApp in Odoo 17 - Odoo ║▌║▌▀Żs



How to Setup WhatsApp in Odoo 17 - Odoo ║▌║▌▀ŻsCeline George
╠²
Integrate WhatsApp into Odoo using the WhatsApp Business API or third-party modules to enhance communication. This integration enables automated messaging and customer interaction management within Odoo 17.APM People Interest Network Conference - Tim Lyons - The neurological levels ...



APM People Interest Network Conference - Tim Lyons - The neurological levels ...Association for Project Management
╠²
APM People Interest Network Conference 2025
-Autonomy, Teams and Tension: Projects under stress
-Tim Lyons
-The neurological levels of
team-working: Harmony and tensions
With a background in projects spanning more than 40 years, Tim Lyons specialised in the delivery of large, complex, multi-disciplinary programmes for clients including Crossrail, Network Rail, ExxonMobil, Siemens and in patent development. His first career was in broadcasting, where he designed and built commercial radio station studios in Manchester, Cardiff and Bristol, also working as a presenter and programme producer. Tim now writes and presents extensively on matters relating to the human and neurological aspects of projects, including communication, ethics and coaching. He holds a MasterŌĆÖs degree in NLP, is an NLP Master Practitioner and International Coach. He is the Deputy Lead for APMŌĆÖs People Interest Network.
Session | The Neurological Levels of Team-working: Harmony and Tensions
Understanding how teams really work at conscious and unconscious levels is critical to a harmonious workplace. This session uncovers what those levels are, how to use them to detect and avoid tensions and how to smooth the management of change by checking you have considered all of them.How to Configure Restaurants in Odoo 17 Point of Sale



How to Configure Restaurants in Odoo 17 Point of SaleCeline George
╠²
Odoo, a versatile and integrated business management software, excels with its robust Point of Sale (POS) module. This guide delves into the intricacies of configuring restaurants in Odoo 17 POS, unlocking numerous possibilities for streamlined operations and enhanced customer experiences.The Story Behind the Abney Park Restoration Project by Tom Walker



The Story Behind the Abney Park Restoration Project by Tom WalkerHistory of Stoke Newington
╠²
Presented at the 24th Stoke Newington History Talks event on 27th Feb 2025
https://stokenewingtonhistory.com/stoke-newington-history-talks/South Hornsey: The Lost Local Authority that Merged with Stoke Newington by T...



South Hornsey: The Lost Local Authority that Merged with Stoke Newington by T...History of Stoke Newington
╠²
Presented at the 24th Stoke Newington History Talks event on 27th Feb 2025
https://stokenewingtonhistory.com/stoke-newington-history-talks/Lesson Plan M1 2024 Lesson Plan M1 2024 Lesson Plan M1 2024 Lesson Plan M1...



Lesson Plan M1 2024 Lesson Plan M1 2024 Lesson Plan M1 2024 Lesson Plan M1...pinkdvil200
╠²
Lesson Plan M1 2024 Lesson Plan M1 2024 Lesson Plan M1 2024 The Battle of Belgrade Road: A WW1 Street Renaming Saga by Amir Dotan



The Battle of Belgrade Road: A WW1 Street Renaming Saga by Amir DotanHistory of Stoke Newington
╠²
Presented at the 24th Stoke Newington History Talks event on 27th Feb 2025
https://stokenewingtonhistory.com/stoke-newington-history-talks/N.C. DPI's 2023 Language Diversity Briefing



N.C. DPI's 2023 Language Diversity BriefingMebane Rash
╠²
The number of languages spoken in NC public schools.How to attach file using upload button Odoo 18



How to attach file using upload button Odoo 18Celine George
╠²
In this slide, weŌĆÖll discuss on how to attach file using upload button Odoo 18. Odoo features a dedicated model, 'ir.attachments,' designed for storing attachments submitted by end users. We can see the process of utilizing the 'ir.attachments' model to enable file uploads through web forms in this slide.Kaun TALHA quiz Prelims - El Dorado 2025



Kaun TALHA quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
╠²
Prelims of Kaun TALHA : a Travel, Architecture, Lifestyle, Heritage and Activism quiz, organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. APM People Interest Network Conference - Tim Lyons - The neurological levels ...



APM People Interest Network Conference - Tim Lyons - The neurological levels ...Association for Project Management
╠²
South Hornsey: The Lost Local Authority that Merged with Stoke Newington by T...



South Hornsey: The Lost Local Authority that Merged with Stoke Newington by T...History of Stoke Newington
╠²
Kaun TALHA quiz Prelims - El Dorado 2025



Kaun TALHA quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
╠²
Solid State Class 12 Chemistry RBSE or CBSE
- 2. ’é× TYPES OF SOLIDS ’é× TYPES OF CRYTALLINE SOLIDS ’é× CRYSTAL LATTICE AND UNIT CELL ’é× CLOSE PACKING AND EFFICIENCY ’é× TYPES VOID ’é× IMPERFECTION IN SOLIDS ’é× ELECTRICAL AND MEGNATIC PROPERTIES OF SOLIDS
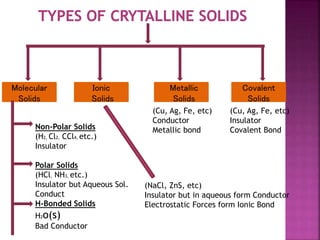
- 3. Molecular Solids Metallic Solids Ionic Solids Covalent Solids Non-Polar Solids (H2, Cl2, CCl4, etc.) Insulator Polar Solids (HCl, NH3, etc.) Insulator but Aqueous Sol. Conduct H-Bonded Solids H2o(s) Bad Conductor (NaCl, ZnS, etc) Insulator but in aqueous form Conductor Electrostatic Forces form Ionic Bond (Cu, Ag, Fe, etc) Conductor Metallic bond (Cu, Ag, Fe, etc) Insulator Covalent Bond
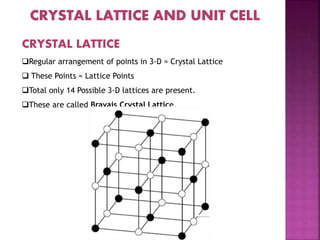
- 4. CRYSTAL LATTICE ’ü▒Regular arrangement of points in 3-D = Crystal Lattice ’ü▒ These Points = Lattice Points ’ü▒Total only 14 Possible 3-D lattices are present. ’ü▒These are called Bravais Crystal Lattice.
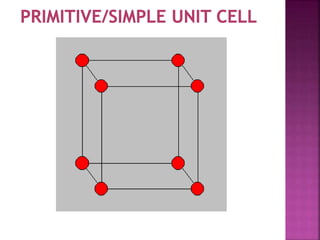
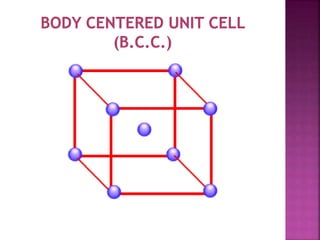
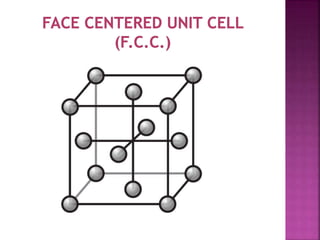
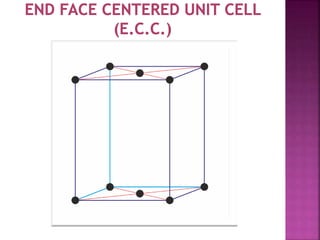
- 5. UNIT CELL ’ü▒ Smallest Portion of Crystal Which is Repeated in different direction and generate the entire lattice called as UNIT CELL. ’ü▒ There are 4 types of unit cell. 1 Primitive/Simple unit Cell 2 Body Centered Unit Cell (B.C.C.) 3 Face Centered Unit Cell (F.C.C.) 4 End Face Centered Unit Cell (E.C.C.)
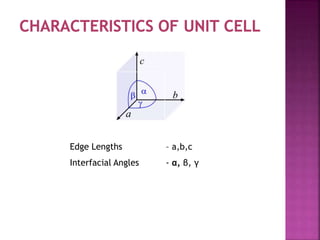
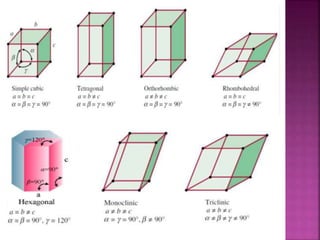
- 10. Edge Lengths ŌĆō a,b,c Interfacial Angles - ╬▒, ╬▓, ╬│
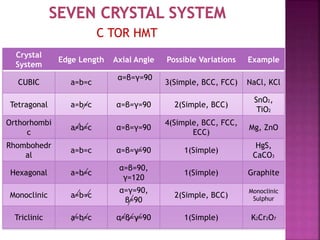
- 11. C TOR HMT Crystal System Edge Length Axial Angle Possible Variations Example CUBIC a=b=c ╬▒=╬▓=╬│=90 3(Simple, BCC, FCC) NaCl, KCl Tetragonal a=b=c ╬▒=╬▓=╬│=90 2(Simple, BCC) SnO2, TiO2 Orthorhombi c a=b=c ╬▒=╬▓=╬│=90 4(Simple, BCC, FCC, ECC) Mg, ZnO Rhombohedr al a=b=c ╬▒=╬▓=╬│=90 1(Simple) HgS, CaCO3 Hexagonal a=b=c ╬▒=╬▓=90, ╬│=120 1(Simple) Graphite Monoclinic a=b=c ╬▒=╬│=90, ╬▓=90 2(Simple, BCC) Monoclinic Sulphur Triclinic a=b=c ╬▒=╬▓=╬│=90 1(Simple) K2Cr2O7







