UNIT I-PART-2.pptx
Download as PPTX, PDF1 like99 views
The document discusses the structure of benzene. When benzene is cooled and x-rayed, all the bond lengths are found to be 0.139nm, making Kekule's alternating single-double bond proposal impossible. Benzene has a molecular formula of C6H6 but does not react like alkenes, undergoing substitution rather than addition reactions. Its stability comes from resonance energy arising from delocalized pi electrons within its hexagonal cyclic structure.
1 of 12
Download to read offline










Recommended
BENZENE AND ITS DERIVATIVES.pptx



BENZENE AND ITS DERIVATIVES.pptxShoaibAhmedAnsari
Ěý
Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma around petrol (gasoline) stations.
It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually.
Although a major industrial chemical, benzene finds limited use in consumer items because of its toxicity
BENZENE AND ITS DERIVATIVES 123.pptx



BENZENE AND ITS DERIVATIVES 123.pptxYogesh Harangule
Ěý
1. Introduction
2. History of benzene
3. Nomenclature
4. Orbital structure
5. Kekule structure
6. Resonance structure
7. Resonance energy and stability 8. Structural evidence
9. Synthetic evidence
10. Analytical evidence
11. Aromaticity and huckle rule
12. Method of preparation of benzene
13. Electrophilic substitution of benzene
14. Classification of substituent 15. Directive effect : Ortho and para director, meta director
16. Reaction of monosubstituted benzene
17. Effect of Substituents on reactivity and orientation of monosubstituted benzene towards electrophilic substitution 18. Structure and uses of BHC,DDT, Saccharine and chloramineBenzene and its Derivatives new (1).pptx



Benzene and its Derivatives new (1).pptxPramod kumar
Ěý
Characteristics of Benzene, Preparation and orientation of reaction Pharmaceutical Organic Chemistry , Jimmy Alexander, Associate Professor Dept...



Pharmaceutical Organic Chemistry , Jimmy Alexander, Associate Professor Dept...JIMMYALEX8
Ěý
This document provides information about benzene and its derivatives. It begins by defining benzene as a naturally occurring and industrially produced aromatic compound with the chemical formula C6H6. It then discusses the structure of benzene, including that it has a planar hexagonal ring structure with alternating single and double bonds between carbon atoms. The document also explains the molecular orbital theory of benzene and how the delocalization of its pi electrons leads to its unusual stability compared to other alkenes. Finally, it provides examples of chemical reactions of benzene including halogenation, nitration, sulfonation, alkylation, acylation, and nucleophilic aromatic substitution.Benzene Pharmaceutical organic chemistry ll unit 1



Benzene Pharmaceutical organic chemistry ll unit 1ToniBlair1
Ěý
B pharmacy 3rd semester unit 1 BenzeneBenzene and its Derivatives new.presentation



Benzene and its Derivatives new.presentationPramod kumar
Ěý
Preparation, reaction ,charactristics of benzeneBenzen lecture



Benzen lectureAshwani96
Ěý
The document discusses the properties and structure of benzene (C6H6). It explains that benzene is planar and has resonance structures with delocalized pi electrons. This delocalization contributes to benzene's unusual stability compared to other unsaturated hydrocarbons. The document also introduces Kekulé structures and describes how current models of benzene are based on resonance and electron delocalization rather than distinct single and double bonds. It discusses how benzene's properties satisfy criteria for aromaticity including being cyclic, planar, fully conjugated, and having 4n+2 pi electrons as per Hückel's rule.Aromatic1



Aromatic1Khilawan Patel
Ěý
In chemistry, aromaticity is a property of cyclic (ring-shaped), planar (flat) structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to other geometric or connective arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability.AROMATIC HYDROCARBON FOR XI & XII.pptx



AROMATIC HYDROCARBON FOR XI & XII.pptxJayNarayanMitruka1
Ěý
The term 'aromatic' was derived from Greek word 'aroma' meaning sweet fragrance.According to
ĚýKekule, benzene and its derivatives are aromatic compounds.Aromatic hydrocarbons are also called ARENES. In this sense, arenes and their derivatives are allĚý'Aromatic compounds'. Aromatic compounds have cyclic & flat structure. They areĚýhighly unsaturated butĚýdo not giveĚý unsaturationĚýtest. Benzene does not discharge pink colour ofĚý alkalineĚýKMnO4Ěýsolution (also known as Baeyer'sĚýreagent)Ěý Ěý ĚýandĚýredĚýcolour of Br2/CCl4.They are stabilizedĚýdue to resonance or due to delocalization ofĚýĎ€-electrons, soĚýthey have low values of heats of hydrogenation than expected. TheyĚýobey Huckel's rule: A conjugated ring system containing (4n+2)Ď€e-ĚýwillĚýshow aromatic character.(where n=0,1,2,3,4,5,etc). The resonance hybrid structure of benzene can explain the unusual property of double bonds in it which causes the extra stability of benzene molecule. The term orientation indicates the assignments of the positions of the substituents with respect to a substituent already present in the ring during electrophilic substitution reaction of a mono substituted benzene.
Benzene and it's derivatives ||Organic chemistry



Benzene and it's derivatives ||Organic chemistryBanny Sv
Ěý
Benzene is a colorless, flammable substance with a sweet odor that is made up of six carbon atoms and one hydrogen atom. It is a major component of gasoline and is used in the manufacturing of many products, including plastics, synthetic fibers, resins, colorants, rubber lubricants, detergents, medications, and pesticides.Basic Introduction Of benzene 



Basic Introduction Of benzene Student
Ěý
(1) The document introduces benzene and its structure as proposed by Kekulé, which involves alternating single and double bonds in a ring of 6 carbon atoms. (2) It discusses benzene's unusual stability arising from resonance between different Lewis structures. (3) The main reactions of benzene are electrophilic aromatic substitutions, such as bromination, which proceeds through formation of an arenium ion intermediate.Benzene and its derivatives- According to PCI Syllabus 



Benzene and its derivatives- According to PCI Syllabus Ganesh Mote
Ěý
Benzene history, nomenclature, orbital structure, resonance structure, kekule structure,synthetic evidences, structural and analytical evidences, Directive effect of benzene, structure and uses of DDT, BHC, saccharine Benzene and its derivatives matriculation sylibus.pptx



Benzene and its derivatives matriculation sylibus.pptxnorwahidahwahabmeb10
Ěý
The document discusses aromatic compounds and benzene derivatives. It defines aromaticity and outlines Hückel's rule for determining aromaticity based on the number of π electrons in a conjugated cyclic system. It describes the structures of benzene proposed by Kekulé and the modern resonance hybrid structure. The document also covers IUPAC nomenclature of substituted benzenes, electrophilic aromatic substitution reactions including nitration, halogenation, and Friedal-Crafts alkylation and acylation. It discusses ortho-para and meta directing substituents and their influence on substitution orientation. B.Sc.FY AROMATICITY Kohinoor College khultabad.ppt



B.Sc.FY AROMATICITY Kohinoor College khultabad.pptNamdeoWaltureGuru
Ěý
This document discusses aromaticity and benzene. It begins by introducing benzene as the simplest aromatic hydrocarbon, noting that it has four degrees of unsaturation but does not readily undergo addition reactions like other unsaturated hydrocarbons. The document then discusses various proposed structures for benzene, including Kekulé structures and modern descriptions based on resonance and delocalization of pi electrons. It introduces Hückel's rule for aromaticity, stating that compounds must be cyclic, planar, completely conjugated, and contain 4n+2 pi electrons. The document provides examples of aromatic and antiaromatic compounds according to this rule. It also discusses nomenclature of benzene derivatives and spectroscopic properties related to aromaticity.Aromatic Hydrocarbon



Aromatic HydrocarbonJayNarayanMitruka
Ěý
The document discusses aromatic hydrocarbons and provides details about benzene. It defines aromatic compounds as those containing benzene or benzenoid rings. Benzene's structure was originally proposed by Kekulé as alternating single and double bonds, but it is now understood to be a resonance hybrid of two contributing structures with delocalized pi electrons. This explains benzene's stability and reactivity toward substitution rather than addition reactions. The document provides examples of aromatic compounds and substitution patterns on benzene derivatives. It also discusses the orientation and directing effects of substituents on benzene during electrophilic substitution.Benzene and its derivatives.ppt



Benzene and its derivatives.pptRAJARAMBAPU COLLEGE OF PHARMACY, KASEGAON
Ěý
according to PCI Syllabus
Subject:-Pharmaceutical Organic Chemistry-II
Class/year:-S.Y.B.Pharmacy
Sem:-IIIStructure of benzene



Structure of benzenegopinathannsriramachandraeduin
Ěý
The document discusses the structure of benzene. It describes Kekulé's proposal in 1865 that benzene has a ring structure with alternating single and double bonds between the six carbon atoms. This resonance structure accounts for benzene's stability and its tendency to undergo substitution rather than addition reactions. The orbital model of benzene involves sp2 hybridization of the carbon atoms and delocalization of the pi electrons above and below the plane of the ring, contributing to its additional stability over cyclohexatriene.Chemistry Of Aromatic Compounds By Dr. Gladys Mokua.



Chemistry Of Aromatic Compounds By Dr. Gladys Mokua.MathewJude
Ěý
şÝşÝߣ that venture deep into Benzene, its nomenclature, Its reactions and Benzene substituents reactions. Hope it will be of help to those who want to know about aromaticity.chapter9 aromatic compounds 12class 



chapter9 aromatic compounds 12class PglKiPgli
Ěý
This document provides an overview of aromatic hydrocarbons. It begins by defining aromatic hydrocarbons and noting that benzene is the simplest aromatic hydrocarbon. The document then discusses the structures of benzene proposed by Kekule and modern concepts of benzene's structure using atomic orbital theory. It explains that benzene has a delocalized pi electron cloud responsible for its stability compared to alternatives like cyclohexatriene. The document also provides background on the preparation, properties, reactions and naming of aromatic hydrocarbons including benzene and its derivatives.Benzene story



Benzene storyJames Midgley
Ěý
Here are 3 key problems scientists faced in explaining the structure and bonding of benzene:
1. Benzene did not react like a typical alkene as expected based on its molecular formula.
2. The thermodynamic stability of benzene was greater than predicted if it contained alternating double and single bonds.
3. The carbon-carbon bond lengths in benzene were equal in length, inconsistent with alternating double and single bonds.
Through continued experimentation using new techniques like STM and theoretical developments like valence bond theory, the current understanding of benzene emerged. Scientists now recognize that benzene's electrons are delocalized through conjugation into a ring above and below the nuclei. This explains benzene's extra stability andCc the structure of benzene



Cc the structure of benzeneVishwanath Agrahari
Ěý
The structure of benzene presented a problem to chemists in the 19th century. While its molecular formula suggested multiple double bonds like other reactive hydrocarbons, benzene was surprisingly unreactive. In 1865, Kekulé proposed a ring structure for benzene with alternating single and double bonds. However, this did not explain its stability and low reactivity. The true structure involves delocalization of electrons across the entire ring, with equal bond lengths between carbon atoms. This delocalized pi-bond system confers unusual stability and reactivity to benzene.Benzene Ring and Aromaticity



Benzene Ring and AromaticityGaurab Roy
Ěý
The document presents a slideshow on the topic of benzene, covering its structure, bonding properties, resonance, nomenclature, reactions, and industrial uses. It discusses benzene's molecular structure and hybridization, how resonance leads to delocalized pi bonding and aromaticity. Examples of benzene's industrial applications include use as a solvent and precursor to other aromatic compounds used in dyes, plastics, drugs, and explosives.Aromatic hydrocarbons



Aromatic hydrocarbonsMartin Brown
Ěý
Benzene is an aromatic hydrocarbon with a planar hexagonal ring structure. Each carbon atom in the ring forms four bonds - one with a hydrogen atom and three sigma bonds with the other carbon atoms in the ring. The sixth valence electron of each carbon is delocalized and shared among all six carbon atoms, giving benzene unusual stability and properties compared to other unsaturated hydrocarbons. Aromatic compounds contain a benzene ring in their structure and include benzene itself along with methylbenzene and ethylbenzene. These aromatic hydrocarbons are liquids that are insoluble in water but soluble in non-polar solvents.Pharmaceutical Organic Chemistry-II Introduction-to-Benzene.pptx



Pharmaceutical Organic Chemistry-II Introduction-to-Benzene.pptxdeepak_musmade
Ěý
Pharmaceutical Organic Chemistry-II Introduction-to-Benzene.pptxSynthetic and Analytical evidence of benzene_0000.pdf



Synthetic and Analytical evidence of benzene_0000.pdfIMRANMULLA44
Ěý
Synthetic Evidence of Benzene: Synthetic evidence refers to the methods used to synthesize or produce benzene in the laboratory, which can help confirm its structure. One classic method is the reduction of phenol (C6H5OH) using zinc dust, which results in the formation of benzene (C6H6). This reaction shows the process by which benzene can be synthesized, supporting its existence and structure.
Analytical Evidence of Benzene: Analytical evidence involves techniques used to study and confirm the structure of benzene. Key methods include:
1. Spectroscopy:
NMR (Nuclear Magnetic Resonance): Shows a characteristic proton NMR pattern with six protons in a symmetrical arrangement, supporting the idea of a regular hexagonal ring structure.
IR (Infrared Spectroscopy): Benzene shows a distinct absorption peak around 1500 cm^-1, corresponding to the C–C stretching vibrations typical of an aromatic ring.
2. Chemical Reactions:
Benzene undergoes electrophilic substitution reactions (e.g., halogenation, nitration) rather than addition reactions, which is a key feature of aromatic compounds. This behavior provides strong evidence of its stability and ring structures
BORAZINE- structure, preparation and properties



BORAZINE- structure, preparation and propertiesMn2555
Ěý
1. Borazine is called inorganic benzene because it has a planar hexagonal structure like benzene with alternating B and N atoms.
2. Borazine undergoes addition reactions readily unlike benzene due to the polarity in its B-N bonds from the difference in electronegativity between B and N atoms.
3. Borazine can be synthesized in the laboratory by heating a mixture of LiBH4 and NH4Cl in vacuum at 230°C, which yields 30% borazine.benzene derivatives.pptx



benzene derivatives.pptxJane756411
Ěý
Benzene undergoes substitution reactions rather than addition reactions. It has a cyclic conjugated structure with six carbon atoms forming a hexagonal ring. Early theories proposed that benzene had alternating single and double bonds (Kekule structure). However, modern evidence shows that all the carbon-carbon bonds in benzene are equal in length, indicating that the structure is best represented by a resonance hybrid of different possible structures with delocalized pi-electrons throughout the ring. This resonance stabilization makes benzene unusually stable and reactive toward substitution rather than addition reactions.UNIT I-PART-7.pptx



UNIT I-PART-7.pptxBabaraj Udugade
Ěý
The document discusses electrophilic aromatic substitution reactions of benzene, including nitration, sulfonation, halogenation, Friedel-Crafts alkylation, and Friedel-Crafts acylation. These reactions involve an electrophilic species attacking the benzene ring. A two-step mechanism is proposed where the electrophile first forms a sigma complex with the benzene ring, generating a positively charged intermediate. This is then followed by a rapid loss of a proton to form the substituted benzene product.UNIT I-PART-9.pptx



UNIT I-PART-9.pptxBabaraj Udugade
Ěý
This document discusses how substituents on benzene rings affect the reactivity and orientation of mono-substituted benzene compounds in electrophilic substitution reactions. It notes that for mono-substituted benzene, three possible constitutional isomers may form depending on whether substitution occurs at the ortho, meta, or para sites. The nature of the substituent influences which isomer is favored, with methoxybenzene producing mainly the para isomer and nitrobenzene favoring the meta isomer. The meta carbocation intermediate is less stable than the ortho intermediate due to inability to delocalize the positive charge through resonance to carbon 1.More Related Content
Similar to UNIT I-PART-2.pptx (20)
Aromatic1



Aromatic1Khilawan Patel
Ěý
In chemistry, aromaticity is a property of cyclic (ring-shaped), planar (flat) structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to other geometric or connective arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability.AROMATIC HYDROCARBON FOR XI & XII.pptx



AROMATIC HYDROCARBON FOR XI & XII.pptxJayNarayanMitruka1
Ěý
The term 'aromatic' was derived from Greek word 'aroma' meaning sweet fragrance.According to
ĚýKekule, benzene and its derivatives are aromatic compounds.Aromatic hydrocarbons are also called ARENES. In this sense, arenes and their derivatives are allĚý'Aromatic compounds'. Aromatic compounds have cyclic & flat structure. They areĚýhighly unsaturated butĚýdo not giveĚý unsaturationĚýtest. Benzene does not discharge pink colour ofĚý alkalineĚýKMnO4Ěýsolution (also known as Baeyer'sĚýreagent)Ěý Ěý ĚýandĚýredĚýcolour of Br2/CCl4.They are stabilizedĚýdue to resonance or due to delocalization ofĚýĎ€-electrons, soĚýthey have low values of heats of hydrogenation than expected. TheyĚýobey Huckel's rule: A conjugated ring system containing (4n+2)Ď€e-ĚýwillĚýshow aromatic character.(where n=0,1,2,3,4,5,etc). The resonance hybrid structure of benzene can explain the unusual property of double bonds in it which causes the extra stability of benzene molecule. The term orientation indicates the assignments of the positions of the substituents with respect to a substituent already present in the ring during electrophilic substitution reaction of a mono substituted benzene.
Benzene and it's derivatives ||Organic chemistry



Benzene and it's derivatives ||Organic chemistryBanny Sv
Ěý
Benzene is a colorless, flammable substance with a sweet odor that is made up of six carbon atoms and one hydrogen atom. It is a major component of gasoline and is used in the manufacturing of many products, including plastics, synthetic fibers, resins, colorants, rubber lubricants, detergents, medications, and pesticides.Basic Introduction Of benzene 



Basic Introduction Of benzene Student
Ěý
(1) The document introduces benzene and its structure as proposed by Kekulé, which involves alternating single and double bonds in a ring of 6 carbon atoms. (2) It discusses benzene's unusual stability arising from resonance between different Lewis structures. (3) The main reactions of benzene are electrophilic aromatic substitutions, such as bromination, which proceeds through formation of an arenium ion intermediate.Benzene and its derivatives- According to PCI Syllabus 



Benzene and its derivatives- According to PCI Syllabus Ganesh Mote
Ěý
Benzene history, nomenclature, orbital structure, resonance structure, kekule structure,synthetic evidences, structural and analytical evidences, Directive effect of benzene, structure and uses of DDT, BHC, saccharine Benzene and its derivatives matriculation sylibus.pptx



Benzene and its derivatives matriculation sylibus.pptxnorwahidahwahabmeb10
Ěý
The document discusses aromatic compounds and benzene derivatives. It defines aromaticity and outlines Hückel's rule for determining aromaticity based on the number of π electrons in a conjugated cyclic system. It describes the structures of benzene proposed by Kekulé and the modern resonance hybrid structure. The document also covers IUPAC nomenclature of substituted benzenes, electrophilic aromatic substitution reactions including nitration, halogenation, and Friedal-Crafts alkylation and acylation. It discusses ortho-para and meta directing substituents and their influence on substitution orientation. B.Sc.FY AROMATICITY Kohinoor College khultabad.ppt



B.Sc.FY AROMATICITY Kohinoor College khultabad.pptNamdeoWaltureGuru
Ěý
This document discusses aromaticity and benzene. It begins by introducing benzene as the simplest aromatic hydrocarbon, noting that it has four degrees of unsaturation but does not readily undergo addition reactions like other unsaturated hydrocarbons. The document then discusses various proposed structures for benzene, including Kekulé structures and modern descriptions based on resonance and delocalization of pi electrons. It introduces Hückel's rule for aromaticity, stating that compounds must be cyclic, planar, completely conjugated, and contain 4n+2 pi electrons. The document provides examples of aromatic and antiaromatic compounds according to this rule. It also discusses nomenclature of benzene derivatives and spectroscopic properties related to aromaticity.Aromatic Hydrocarbon



Aromatic HydrocarbonJayNarayanMitruka
Ěý
The document discusses aromatic hydrocarbons and provides details about benzene. It defines aromatic compounds as those containing benzene or benzenoid rings. Benzene's structure was originally proposed by Kekulé as alternating single and double bonds, but it is now understood to be a resonance hybrid of two contributing structures with delocalized pi electrons. This explains benzene's stability and reactivity toward substitution rather than addition reactions. The document provides examples of aromatic compounds and substitution patterns on benzene derivatives. It also discusses the orientation and directing effects of substituents on benzene during electrophilic substitution.Benzene and its derivatives.ppt



Benzene and its derivatives.pptRAJARAMBAPU COLLEGE OF PHARMACY, KASEGAON
Ěý
according to PCI Syllabus
Subject:-Pharmaceutical Organic Chemistry-II
Class/year:-S.Y.B.Pharmacy
Sem:-IIIStructure of benzene



Structure of benzenegopinathannsriramachandraeduin
Ěý
The document discusses the structure of benzene. It describes Kekulé's proposal in 1865 that benzene has a ring structure with alternating single and double bonds between the six carbon atoms. This resonance structure accounts for benzene's stability and its tendency to undergo substitution rather than addition reactions. The orbital model of benzene involves sp2 hybridization of the carbon atoms and delocalization of the pi electrons above and below the plane of the ring, contributing to its additional stability over cyclohexatriene.Chemistry Of Aromatic Compounds By Dr. Gladys Mokua.



Chemistry Of Aromatic Compounds By Dr. Gladys Mokua.MathewJude
Ěý
şÝşÝߣ that venture deep into Benzene, its nomenclature, Its reactions and Benzene substituents reactions. Hope it will be of help to those who want to know about aromaticity.chapter9 aromatic compounds 12class 



chapter9 aromatic compounds 12class PglKiPgli
Ěý
This document provides an overview of aromatic hydrocarbons. It begins by defining aromatic hydrocarbons and noting that benzene is the simplest aromatic hydrocarbon. The document then discusses the structures of benzene proposed by Kekule and modern concepts of benzene's structure using atomic orbital theory. It explains that benzene has a delocalized pi electron cloud responsible for its stability compared to alternatives like cyclohexatriene. The document also provides background on the preparation, properties, reactions and naming of aromatic hydrocarbons including benzene and its derivatives.Benzene story



Benzene storyJames Midgley
Ěý
Here are 3 key problems scientists faced in explaining the structure and bonding of benzene:
1. Benzene did not react like a typical alkene as expected based on its molecular formula.
2. The thermodynamic stability of benzene was greater than predicted if it contained alternating double and single bonds.
3. The carbon-carbon bond lengths in benzene were equal in length, inconsistent with alternating double and single bonds.
Through continued experimentation using new techniques like STM and theoretical developments like valence bond theory, the current understanding of benzene emerged. Scientists now recognize that benzene's electrons are delocalized through conjugation into a ring above and below the nuclei. This explains benzene's extra stability andCc the structure of benzene



Cc the structure of benzeneVishwanath Agrahari
Ěý
The structure of benzene presented a problem to chemists in the 19th century. While its molecular formula suggested multiple double bonds like other reactive hydrocarbons, benzene was surprisingly unreactive. In 1865, Kekulé proposed a ring structure for benzene with alternating single and double bonds. However, this did not explain its stability and low reactivity. The true structure involves delocalization of electrons across the entire ring, with equal bond lengths between carbon atoms. This delocalized pi-bond system confers unusual stability and reactivity to benzene.Benzene Ring and Aromaticity



Benzene Ring and AromaticityGaurab Roy
Ěý
The document presents a slideshow on the topic of benzene, covering its structure, bonding properties, resonance, nomenclature, reactions, and industrial uses. It discusses benzene's molecular structure and hybridization, how resonance leads to delocalized pi bonding and aromaticity. Examples of benzene's industrial applications include use as a solvent and precursor to other aromatic compounds used in dyes, plastics, drugs, and explosives.Aromatic hydrocarbons



Aromatic hydrocarbonsMartin Brown
Ěý
Benzene is an aromatic hydrocarbon with a planar hexagonal ring structure. Each carbon atom in the ring forms four bonds - one with a hydrogen atom and three sigma bonds with the other carbon atoms in the ring. The sixth valence electron of each carbon is delocalized and shared among all six carbon atoms, giving benzene unusual stability and properties compared to other unsaturated hydrocarbons. Aromatic compounds contain a benzene ring in their structure and include benzene itself along with methylbenzene and ethylbenzene. These aromatic hydrocarbons are liquids that are insoluble in water but soluble in non-polar solvents.Pharmaceutical Organic Chemistry-II Introduction-to-Benzene.pptx



Pharmaceutical Organic Chemistry-II Introduction-to-Benzene.pptxdeepak_musmade
Ěý
Pharmaceutical Organic Chemistry-II Introduction-to-Benzene.pptxSynthetic and Analytical evidence of benzene_0000.pdf



Synthetic and Analytical evidence of benzene_0000.pdfIMRANMULLA44
Ěý
Synthetic Evidence of Benzene: Synthetic evidence refers to the methods used to synthesize or produce benzene in the laboratory, which can help confirm its structure. One classic method is the reduction of phenol (C6H5OH) using zinc dust, which results in the formation of benzene (C6H6). This reaction shows the process by which benzene can be synthesized, supporting its existence and structure.
Analytical Evidence of Benzene: Analytical evidence involves techniques used to study and confirm the structure of benzene. Key methods include:
1. Spectroscopy:
NMR (Nuclear Magnetic Resonance): Shows a characteristic proton NMR pattern with six protons in a symmetrical arrangement, supporting the idea of a regular hexagonal ring structure.
IR (Infrared Spectroscopy): Benzene shows a distinct absorption peak around 1500 cm^-1, corresponding to the C–C stretching vibrations typical of an aromatic ring.
2. Chemical Reactions:
Benzene undergoes electrophilic substitution reactions (e.g., halogenation, nitration) rather than addition reactions, which is a key feature of aromatic compounds. This behavior provides strong evidence of its stability and ring structures
BORAZINE- structure, preparation and properties



BORAZINE- structure, preparation and propertiesMn2555
Ěý
1. Borazine is called inorganic benzene because it has a planar hexagonal structure like benzene with alternating B and N atoms.
2. Borazine undergoes addition reactions readily unlike benzene due to the polarity in its B-N bonds from the difference in electronegativity between B and N atoms.
3. Borazine can be synthesized in the laboratory by heating a mixture of LiBH4 and NH4Cl in vacuum at 230°C, which yields 30% borazine.benzene derivatives.pptx



benzene derivatives.pptxJane756411
Ěý
Benzene undergoes substitution reactions rather than addition reactions. It has a cyclic conjugated structure with six carbon atoms forming a hexagonal ring. Early theories proposed that benzene had alternating single and double bonds (Kekule structure). However, modern evidence shows that all the carbon-carbon bonds in benzene are equal in length, indicating that the structure is best represented by a resonance hybrid of different possible structures with delocalized pi-electrons throughout the ring. This resonance stabilization makes benzene unusually stable and reactive toward substitution rather than addition reactions.More from Babaraj Udugade (9)
UNIT I-PART-7.pptx



UNIT I-PART-7.pptxBabaraj Udugade
Ěý
The document discusses electrophilic aromatic substitution reactions of benzene, including nitration, sulfonation, halogenation, Friedel-Crafts alkylation, and Friedel-Crafts acylation. These reactions involve an electrophilic species attacking the benzene ring. A two-step mechanism is proposed where the electrophile first forms a sigma complex with the benzene ring, generating a positively charged intermediate. This is then followed by a rapid loss of a proton to form the substituted benzene product.UNIT I-PART-9.pptx



UNIT I-PART-9.pptxBabaraj Udugade
Ěý
This document discusses how substituents on benzene rings affect the reactivity and orientation of mono-substituted benzene compounds in electrophilic substitution reactions. It notes that for mono-substituted benzene, three possible constitutional isomers may form depending on whether substitution occurs at the ortho, meta, or para sites. The nature of the substituent influences which isomer is favored, with methoxybenzene producing mainly the para isomer and nitrobenzene favoring the meta isomer. The meta carbocation intermediate is less stable than the ortho intermediate due to inability to delocalize the positive charge through resonance to carbon 1.UNIT I-PART-1.pptx



UNIT I-PART-1.pptxBabaraj Udugade
Ěý
Benzene's structure was originally proposed to have alternating single and double bonds by Kekulé, but this posed several problems. Benzene is more stable than expected from the Kekulé structure and undergoes substitution reactions rather than addition reactions like ethene. The bonds in benzene are also all the same length, unlike what would be expected from alternating single and double bonds. Modern evidence shows benzene has a delocalized pi electron cloud above and below the ring that contributes to its unusual stability and reactivity.UNIT I-PART-6.pptx



UNIT I-PART-6.pptxBabaraj Udugade
Ěý
Benzene and its derivatives can undergo various reactions including nitration, sulphonation, and halogenation which introduce nitro, sulfo, and halo groups onto the benzene ring. The Friedel-Crafts alkylation reaction can add alkyl groups to benzene but has limitations. Friedel-Crafts acylation can similarly add acyl groups to benzene.UNIT I-PART-8.pptx



UNIT I-PART-8.pptxBabaraj Udugade
Ěý
The document discusses how substituents on benzene rings can influence the reactivity and product distribution of electrophilic substitution reactions. It states that electron-donating groups like hydroxyl and methoxy increase reactivity by thousands of times, while electron-withdrawing groups like nitro decrease reactivity by millions. This is due to the inductive effect of substituents. It also discusses how conjugation allows substituents to donate or withdraw electrons through resonance. Finally, it explains that substituents can direct reaction to the ortho, meta, or para positions based on the stability of the carbocation intermediates.UNIT I-PART-3.pptx



UNIT I-PART-3.pptxBabaraj Udugade
Ěý
The orbital structure of benzene consists of six carbon atoms in a ring, with each carbon atom containing one unhybridized p orbital perpendicular to the plane of the ring. These p orbitals overlap side-to-side between adjacent carbon atoms, forming two delocalized pi molecular orbitals above and below the plane of the ring. This delocalization of the pi electrons imparts unique stability to the benzene molecule. The pi molecular orbital diagram of benzene contains six pi orbitals arising from the six carbon p orbitals, with the lowest energy orbitals having zero nodes and the highest energy orbital having three nodes dividing the ring into three sections.UNIT I-PART-5.pptx



UNIT I-PART-5.pptxBabaraj Udugade
Ěý
Benzene exhibits resonance between two contributing structures that differ in the position of the double bonds. This resonance delocalizes the pi electrons in the benzene ring and makes the molecule extremely stable despite having alternating single and double bonds. For a molecule to be considered aromatic, it must meet four conditions - it must be cyclic, have conjugation between every atom in the ring, contain 4n+2 pi electrons, and be flat. These conditions allow for the stabilization gained from resonance throughout the pi electron system.UNIT I-PART-10.pptx



UNIT I-PART-10.pptxBabaraj Udugade
Ěý
This document provides information on the chemical structures, properties, and uses of 4 compounds:
1) DDT is a synthetic pesticide that was widely used but is now limited due to toxicity. It affects the nervous system.
2) Saccharin is an artificial sweetener used as a food additive. It is non-metabolized and suitable for diabetics.
3) Benzene hexachloride is an insecticide used on crops and in pharmaceutical products to treat lice and scabies.
4) Chloramine is an organic sodium salt used as a disinfectant and antifouling biocide.UNIT I-PART-4.pptx



UNIT I-PART-4.pptxBabaraj Udugade
Ěý
Hückel's rule states that a planar cyclic molecule with 4n + 2 π electrons will be aromatic. The rule is used to determine if a molecule is aromatic, antiaromatic, or nonaromatic based on its number of electrons and structure. Benzene, with 6 π electrons, is an example of an aromatic molecule that follows Hückel's rule of 4n + 2 electrons, with n=1. The stability of aromatic molecules is due to all bonding molecular orbitals being filled with electrons, while no antibonding orbitals are occupied.Recently uploaded (20)
Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Finals of Rass MELAI : a Music, Entertainment, Literature, Arts and Internet Culture Quiz organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. N.C. DPI's 2023 Language Diversity Briefing



N.C. DPI's 2023 Language Diversity BriefingMebane Rash
Ěý
The number of languages spoken in NC public schools.How to Configure Flexible Working Schedule in Odoo 18 Employee



How to Configure Flexible Working Schedule in Odoo 18 EmployeeCeline George
Ěý
In this slide, we’ll discuss on how to configure flexible working schedule in Odoo 18 Employee module. In Odoo 18, the Employee module offers powerful tools to configure and manage flexible working schedules tailored to your organization's needs.The Constitution, Government and Law making bodies .



The Constitution, Government and Law making bodies .saanidhyapatel09
Ěý
This PowerPoint presentation provides an insightful overview of the Constitution, covering its key principles, features, and significance. It explains the fundamental rights, duties, structure of government, and the importance of constitutional law in governance. Ideal for students, educators, and anyone interested in understanding the foundation of a nation’s legal framework.
How to Modify Existing Web Pages in Odoo 18



How to Modify Existing Web Pages in Odoo 18Celine George
Ěý
In this slide, we’ll discuss on how to modify existing web pages in Odoo 18. Web pages in Odoo 18 can also gather user data through user-friendly forms, encourage interaction through engaging features. The Dravidian Languages: Tamil, Telugu, Kannada, Malayalam, Brahui, Kuvi, Tulu



The Dravidian Languages: Tamil, Telugu, Kannada, Malayalam, Brahui, Kuvi, TuluDrIArulAram
Ěý
The Dravidian Languages by Arul AramKaun TALHA quiz Prelims - El Dorado 2025



Kaun TALHA quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Prelims of Kaun TALHA : a Travel, Architecture, Lifestyle, Heritage and Activism quiz, organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. Information Technology for class X CBSE skill Subject



Information Technology for class X CBSE skill SubjectVEENAKSHI PATHAK
Ěý
These questions are based on cbse booklet for 10th class information technology subject code 402. these questions are sufficient for exam for first lesion. This subject give benefit to students and good marks. if any student weak in one main subject it can replace with these marks.Adventure Activities Final By H R Gohil Sir



Adventure Activities Final By H R Gohil SirGUJARATCOMMERCECOLLE
Ěý
Adventure Activities Final By H R Gohil SirHow to use Init Hooks in Odoo 18 - Odoo şÝşÝߣs



How to use Init Hooks in Odoo 18 - Odoo şÝşÝߣsCeline George
Ěý
In this slide, we’ll discuss on how to use Init Hooks in Odoo 18. In Odoo, Init Hooks are essential functions specified as strings in the __init__ file of a module.EDL 290F Week 3 - Mountaintop Views (2025).pdf



EDL 290F Week 3 - Mountaintop Views (2025).pdfLiz Walsh-Trevino
Ěý
EDL 290F Week 3 - Mountaintop Views (2025).pdfA PPT Presentation on The Princess and the God: A tale of ancient India by A...



A PPT Presentation on The Princess and the God: A tale of ancient India by A...Beena E S
Ěý
A PPT Presentation on The Princess and the God: A tale of ancient India by Aaron ShepardUseful environment methods in Odoo 18 - Odoo şÝşÝߣs



Useful environment methods in Odoo 18 - Odoo şÝşÝߣsCeline George
Ěý
In this slide we’ll discuss on the useful environment methods in Odoo 18. In Odoo 18, environment methods play a crucial role in simplifying model interactions and enhancing data processing within the ORM framework.Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Prelims of Rass MELAI : a Music, Entertainment, Literature, Arts and Internet Culture Quiz organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Kaun TALHA quiz Prelims - El Dorado 2025



Kaun TALHA quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
UNIT I-PART-2.pptx
- 1. 1 UNIT I: Benzene and its derivatives Analytical, synthetic and other evidences in the derivation of structure of benzene
- 2. 2
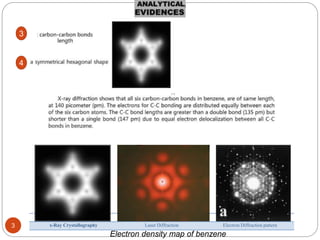
- 3. 3 x-Ray Crystallography Laser Diffraction Electron Diffraction pattern Electron density map of benzene 3 4
- 4. 4 When benzene is cooled it crystallizes and x-ray diffraction can be used to measure the bond lengths, which in benzene are all found to be 0.139nm. Therefore, Kekule’s proposal of alternating single and double bonds is impossible. 5
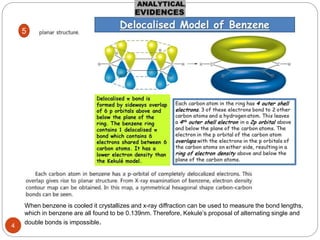
- 5. 1 Not a Straight Chain
- 7. 3 C=C 3 3
- 8. 8 ď‚— Molecular formula of benzene is C6 H6 , it seemed clear that the molecule must be highly unsaturated ď‚— benzene does not show the chemical properties of alkenes ď‚— Benzene does undergo chemical reactions, but its characteristic reaction is substitution rather than addition. ď‚— When benzene is treated with bromine in the presence of ferric chloride as a catalyst, for example, only one compound with the molecular formula C6 H5 Br forms ď‚— Chemists concluded, therefore, that all six carbons and all six hydrogen's of benzene must be equivalent. 4
- 9. There are mainly three factors that decide the overall stability of benzene over other organic compounds. These factors are as follows: Resonance energy Aromatic stability Structural stability Resonance hybrid Resonance Resonance energy Stability 1 Presence of pi(4n+2)Ď€ electrons. Presence of delocalized electron Hexagonal in shape: less angle
- 10. 10 Stability 1
- 12. 12 Summary






