X-Ray Diffraction technique Introduction Working Principal Application.pptx
- 1. X – RAY DIFFRACTION (XRD) Introduction, Working principal, Applications
- 2. Introduction  It is a novel & non destructive method of chemical analysis and a variety of x –ray techniques are available in practice.  These are : X – Ray Absorption : X-ray diffraction X-ray Fluorescence  X – ray diffraction “ Every crystalline substance gives a pattern; the same substance always gives the same pattern; and in a mixture of substances each produces its pattern independently of the others”  The X-ray diffraction pattern of a pure substance is, therefore, like a fingerprint of the substance. It is based on the scattering of x-rays by crystals.  Definition The atomic planes of a crystal cause an incident beam of X-rays to interfere with one another as they leave the crystal. The phenomenon is called X-ray diffraction.
- 3. What is X-ray Diffraction ?
- 4. Why XRD?  Measure the average spacing's between layers or rows of atoms  Determine the orientation of a single crystal or grain  Find the crystal structure of an unknown material  Measure the size, shape and internal stress of small crystalline regions
- 5. Effect of sample thickness on the absorption of X-rays diffracted beam film incident beam crystal
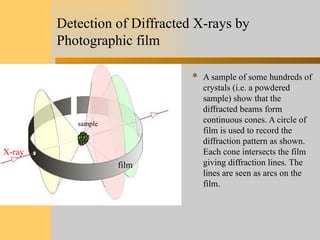
- 6. Detection of Diffracted X-rays by Photographic film  A sample of some hundreds of crystals (i.e. a powdered sample) show that the diffracted beams form continuous cones. A circle of film is used to record the diffraction pattern as shown. Each cone intersects the film giving diffraction lines. The lines are seen as arcs on the film. sample film X-ray
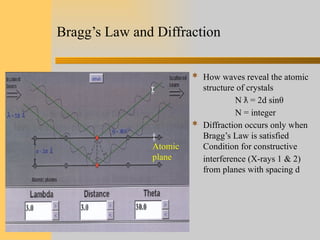
- 7. Bragg’s Law and Diffraction  How waves reveal the atomic structure of crystals N = 2d sin ƛ θ N = integer  Diffraction occurs only when Bragg’s Law is satisfied Condition for constructive interference (X-rays 1 & 2) from planes with spacing d Atomic plane
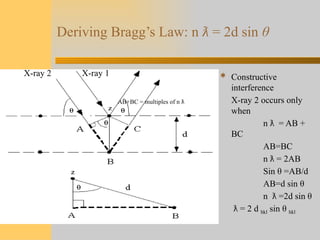
- 8. Deriving Bragg’s Law: n ƛ = 2d sin θ  Constructive interference X-ray 2 occurs only when n = AB + ƛ BC AB=BC n = 2AB ƛ Sin θ =AB/d AB=d sin θ n =2d sin ƛ θ = 2 d ƛ hkl sin θ hkl X-ray 2 X-ray 1 AB+BC = multiples of n ƛ
- 9. Planes in Crystals-2 dimension  Different planes have different spacing  To satisfy Bragg’s Law, q must change as d changes e.g., q decreases as d increases.
- 10. Basics of Crystallography  The atoms are arranged in a regular pattern, and there is as smallest volume element that by repetition in three dimensions describes the crystal. This smallest volume element is called a unit cell.  Crystals consist of planes of atoms that are spaced a distance d apart, but can be resolved into many atomic planes, each with a different d spacing.  The dimensions of the unit cell is described by three axes : a, b, c and the angles between them α, β , and γ are lattice constants which can be determined by XRD. Lattice
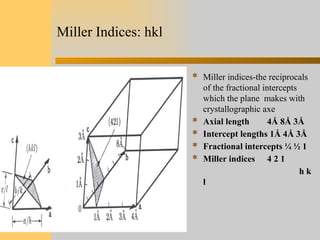
- 11. Miller Indices: hkl  Miller indices-the reciprocals of the fractional intercepts which the plane makes with crystallographic axe  Axial length 4Å 8Å 3Å  Intercept lengths 1Å 4Å 3Å  Fractional intercepts ¼ ½ 1  Miller indices 4 2 1 h k l
- 12. Production of X-rays  X-rays are produced whenever high-speed electrons collide with a metal target.  A source of electrons – hot W filament, a high accelerating voltage between the cathode (W) and the anode and a metal target, Cu, Al, Mo, Mg.  The anode is a water-cooled block of Cu containing desired target metal.
- 13. Specimen Preparation  Powders: 0.1μm < particle size < 40 μm Peak broadening less diffraction occurring  Bulks: smooth surface after polishing, specimens should be thermal annealed to eliminate any surface deformation induced during polishing.
- 14. A Modern Automated X-ray Diffractometer X-ray Tube Detector Sample stage θ θ2 Cost: $560K to 1.6M
- 15. Basic components & Features of XRD  Production  Diffraction  Detection  Interpretation
- 16. Detection of Diffracted X-rays by a Diffractometer Bragg - Brentano Focus Geometry, Cullity
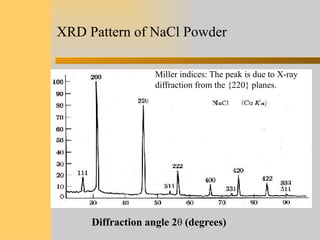
- 17. XRD Pattern of NaCl Powder Diffraction angle 2θ (degrees) Miller indices: The peak is due to X-ray diffraction from the {220} planes.
- 18. Significance of Peak Shape in XRD  Peak position  Peak width  Peak intensity Important for  Particle or  grain size  Residual strain Can also be fit with Gaussian, Lerentzian, Gaussian-Lerentzian etc.
- 19. Effect of Lattice Strain on Diffraction Peak Position and Width No Strain Uniform Strain (d1-do)/do Peak moves, no shape changes Non-uniform Strain D1 =/constant Peak broadens Shifts to lower angles Exceeds d0 on top, smaller than d0 on the bottom
- 20. Applications of XRD  XRD is a non destructive technique to identify crystalline phases and orientation - Obtain XRD pattern ; Measure d-spacings ; Obtain integrated intensities ; - Compare data with known standards in the JCPDS file  To determine structural properties: - Lattice parameters (10-4Å),, grain size, expitaxy, phase composition, prefer strained orientation (Laue) order-disorder transformation, thermal expansion  To measure thickness of thin films and multi-layers*  To determine atomic arrangement  Detection limits: ~3% in a two phase mixture; can be ~0.1% with synchrotron radiation Spatial resolution: normally none
- 21. Applications of XRD  The electron density and accordingly, the position of the atoms in complex structures, such as penicillin may be determined from a comprehensive mathematical study of the x-ray diffraction pattern.  The elucidation of structure of penicillin by xrd paved the way for the later synthesis of penicillin.  The powder xrd pattern may be thought of as finger print of the single crystal structure, and it may be used conduct qualitative and quantitative analysis.  Xrd can also be used to determine whether the compound is solvated or not
- 22. Applications of XRD  Particle size determination by applying the relation. v= V. δθ. cos θ / 2n Where v = the volume or size of an individual crystalline V= the total volume of the specimen irradiated n = the number of spots in a deffraction ring at a Bragg angle θ δθ = the divergence of the X –ray beam  Determination of Cis-Trans isomerism  It is used to assess the weathering and degradation of natural and synthetic , minerals.  Tooth enamel and dentine have been examined by xrd.  State of anneal in metals