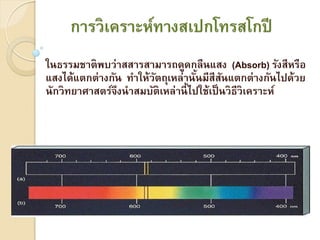
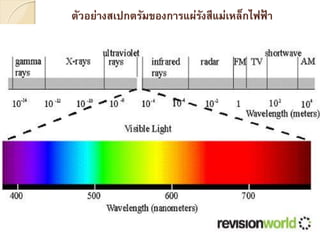
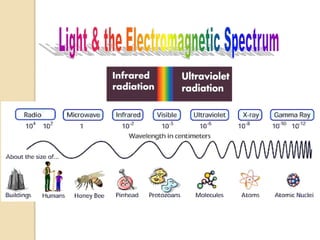
ÓĖüÓĖ▓ÓĖŻÓĖ¦ÓĖ┤Ó╣ĆÓĖäÓĖŻÓĖ▓ÓĖ░ÓĖ½Ó╣īÓĖŚÓĖ▓ÓĖćÓĖ¬Ó╣Č─ÓĖøÓĖüÓ╣éÓĖŚÓĖŻÓĖ¬Ó╣éÓĖüÓĖøÓĖĄÓ╣āÓĖŖÓ╣ēÓĖłÓĖŻÓĖ┤ÓĖć1
- 1. ÓĖüÓĖ▓ÓĖŻÓĖ¦ÓĖ┤Ó╣ĆÓĖäÓĖŻÓĖ▓ÓĖ░ÓĖ½Ó╣īÓĖŚÓĖ▓ÓĖćÓĖ¬Ó╣ĆÓĖøÓĖüÓ╣éÓĖŚÓĖŻÓĖ¬Ó╣éÓĖüÓĖøÓĖĄ Ó╣āÓĖÖÓĖśÓĖŻÓĖŻÓĖĪÓĖŖÓĖ▓ÓĖĢÓĖ┤ÓĖ×ÓĖÜÓĖ¦Ó╣łÓĖ▓ÓĖ¬ÓĖ¬ÓĖ▓ÓĖŻÓĖ¬ÓĖ▓ÓĖĪÓĖ▓ÓĖŻÓĖ¢ÓĖöÓĖ╣ÓĖöÓĖüÓĖźÓĖĘÓĖÖÓ╣üÓĖ¬ÓĖć (Absorb) ÓĖŻÓĖ▒ÓĖćÓĖ¬ÓĖĄÓĖ½ÓĖŻÓĖĘÓĖŁ Ó╣üÓĖ¬ÓĖćÓ╣äÓĖöÓ╣ēÓ╣üÓĖĢÓĖüÓĖĢÓ╣łÓĖ▓ÓĖćÓĖüÓĖ▒ÓĖÖ ÓĖŚÓĖ▓Ó╣āÓĖ½Ó╣ēÓĖ¦ÓĖĢÓĖ¢ÓĖĖÓ╣ĆÓĖ½ÓĖźÓ╣łÓĖ▓ÓĖÖÓĖ▒ÓĖÖÓĖĪÓĖĄÓĖ¬ÓĖĄÓĖ¬ÓĖÖÓ╣üÓĖĢÓĖüÓĖĢÓ╣łÓĖ▓ÓĖćÓĖüÓĖ▒ÓĖÖÓ╣äÓĖøÓĖöÓ╣ēÓĖ¦ÓĖó ÓĖ▒ Ó╣ē ÓĖ▒ ÓĖÖÓĖ▒ÓĖüÓĖ¦ÓĖ┤ÓĖŚÓĖóÓĖ▓ÓĖ©ÓĖ▓ÓĖ¬ÓĖĢÓĖŻÓ╣īÓĖłÓĖČÓĖćÓĖÖÓĖ▓ÓĖ¬ÓĖĪÓĖÜÓĖ▒ÓĖĢÓĖ┤Ó╣ĆÓĖ½ÓĖźÓ╣łÓĖ▓ÓĖÖÓĖĄÓ╣ē Ó╣äÓĖøÓ╣āÓĖŖÓ╣ēÓ╣ĆÓĖøÓ╣ć ÓĖÖÓĖ¦ÓĖ┤ÓĖśÓĖĄÓĖ¦ÓĖ┤Ó╣ĆÓĖäÓĖŻÓĖ▓ÓĖ░ÓĖ½Ó╣ī
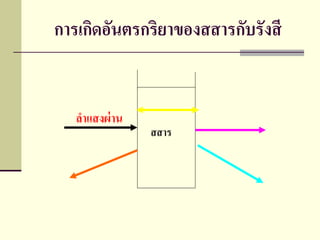
- 2. ÓĖ¬Ó╣ĆÓĖøÓĖüÓ╣éÓĖŚÓĖŻÓĖ¬Ó╣éÓĖäÓĖøÓĖĄ ’ü«ÓĖ½ÓĖĪÓĖ▓ÓĖóÓĖ¢ÓĖČÓĖć ÓĖüÓĖ▓ÓĖŻÓ╣üÓĖóÓĖü ÓĖüÓĖ▓ÓĖŻÓĖĢÓĖŻÓĖ¦ÓĖłÓĖ¬ÓĖŁÓĖÜ Ó╣üÓĖźÓĖ░ÓĖüÓĖ▓ÓĖŻ ÓĖÜÓĖ▒ÓĖÖÓĖŚÓĖČÓĖüÓĖ×ÓĖźÓĖ▒ÓĖćÓĖćÓĖ▓ÓĖÖÓĖŚÓĖĄÓ╣ĆÓ╣ł ÓĖøÓĖźÓĖĄÓĖóÓĖÖÓ╣äÓĖøÓ╣ĆÓĖüÓĖĄÓĖóÓĖ¦ÓĖüÓĖ▒ÓĖÜÓĖÖÓĖ┤ÓĖ¦Ó╣ĆÓĖäÓĖźÓĖĄÓĖóÓĖ¬ Ó╣ł Ó╣ł ÓĖŁÓĖ░ÓĖĢÓĖŁÓĖĪ Ó╣äÓĖŁÓĖŁÓĖŁÓĖÖ ÓĖ½ÓĖŻÓĖĘÓĖŁÓ╣éÓĖĪÓ╣ĆÓĖźÓĖüÓĖĖÓĖź ’ü«ÓĖ×ÓĖźÓĖ▒ÓĖćÓĖćÓĖ▓ÓĖÖÓĖŚÓĖĄÓ╣ĆÓ╣ł ÓĖøÓĖźÓĖĄÓĖóÓĖÖÓ╣äÓĖøÓĖÖÓĖ▒Ó╣ēÓĖÖÓ╣ĆÓĖÖÓĖĘÓ╣łÓĖŁÓĖćÓĖłÓĖ▓ÓĖüÓĖüÓĖ▓ÓĖŻÓ╣ĆÓĖüÓĖ┤ÓĖö ÓĖŁÓĖĄÓĖĪÓĖ┤ÓĖ¬ Ó╣ł ÓĖŖÓĖ▒ÓĖÖ(Emission) ÓĖüÓĖ▓ÓĖŻÓĖöÓĖ╣ÓĖöÓĖüÓĖźÓĖĘÓĖÖ (Absorption) ÓĖüÓĖ▓ÓĖŻ ÓĖüÓĖŻÓĖ░Ó╣ĆÓĖłÓĖ┤ÓĖć(Scattering)
- 3. ÓĖüÓĖ▓ÓĖŻÓ╣ĆÓĖüÓĖ┤ÓĖČŽĖŁÓĖ▒ÓĖÖÓĖĢÓĖŻÓĖüÓĖŻÓĖ┤ÓĖóÓĖ▓ÓŠ║ÓĖŁÓĖćÓĖ¬ÓĖ¬ÓĖ▓ÓĖŻÓĖüÓĖ▒ÓĖÜÓĖŻÓĖ▒ÓĖćÓĖ¬ÓĖĄ ÓĖźÓĖ▓Ó╣üÓĖ¬ÓĖćÓĖ£Ó╣ł ÓĖ▓ÓĖÖ ÓĖ¬ÓĖ¬ÓĖ▓ÓĖŻ
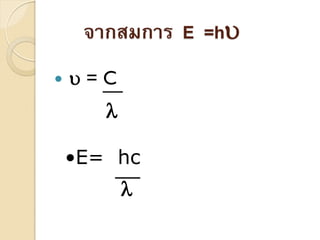
- 4. ÓĖüÓĖ▓ÓĖŻÓ╣üÓĖ£Ó╣łÓĖŻÓĖćÓĖ¬ÓĖĄÓ╣üÓĖĪÓ╣łÓ╣ĆÓĖ½ÓĖźÓ╣ćÓĖüÓ╣äÓĖ¤ÓĖ¤Ó╣ē ÓĖ▓ÓĖ½ÓĖŻÓĖĘÓĖŁÓ╣üÓĖ¬ÓĖć ÓĖ▒ ’éŚÓĖüÓĖ▓ÓĖŻÓ╣üÓĖ£Ó╣łÓĖŻÓĖćÓĖ¬ÓĖĄÓĖéÓĖŁÓĖćÓ╣üÓĖĪÓ╣łÓ╣ĆÓĖ½ÓĖźÓ╣ćÓĖüÓ╣äÓĖ¤ÓĖ¤Ó╣ēÓĖ▓ÓĖłÓĖ▒ÓĖöÓ╣ĆÓĖøÓ╣ć ÓĖÖÓĖ×ÓĖźÓĖ▒ÓĖćÓĖćÓĖ▓ÓĖÖÓĖŻÓĖ╣ÓĖø ÓĖ▒ ÓĖ½ÓĖÖÓĖČÓ╣łÓĖć ’éŚÓĖŁÓĖóÓĖ╣Ó╣āÓĖÖÓĖźÓĖ▒ÓĖüÓĖ®ÓĖōÓĖ░ÓĖŚÓĖĄÓ╣ĆÓĖøÓ╣ć ÓĖÖÓĖŁÓĖÖÓĖĖ ÓĖĀÓĖ▓ÓĖä ÓĖŚÓĖĄÓ╣ĆÓĖŻÓĖĄÓĖóÓĖüÓĖ¦Ó╣łÓĖ▓Ó╣éÓĖ¤ÓĖĢÓĖŁÓĖÖ Ó╣ł Ó╣ł Ó╣ł ’éŚÓĖ×ÓĖźÓĖ▒ÓĖćÓĖćÓĖ▓ÓĖÖÓ╣éÓĖ¤ÓĖĢÓĖŁÓĖÖÓĖłÓĖ░Ó╣ĆÓĖøÓ╣ć ÓĖÖÓĖøÓĖÅÓĖ┤ÓĖĀÓĖ▓ÓĖäÓ╣éÓĖöÓĖóÓĖĢÓĖŻÓĖćÓĖüÓĖ▒ÓĖÜÓĖäÓĖ¦ÓĖ▓ÓĖĪÓĖ¢ÓĖĄÓ╣ł ’ü¼ ’éŚÓĖ¬ÓĖĪÓĖüÓĖ▓ÓĖŻÓĖäÓĖĘÓĖŁ E =h’üĄ
- 5. ÓĖłÓĖ▓ÓĖüÓĖ¬ÓĖĪÓĖüÓĖ▓ÓĖŻ E =h’üĄ ’éŚ ’üĄ=C ’ü¼ ŌĆóE= hc ’ü¼
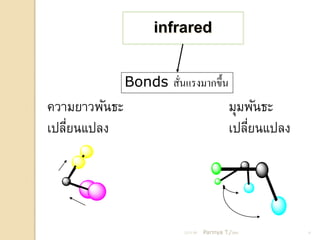
- 8. infrared Bonds ÓĖ¬ÓĖ▒ÓĖÖÓ╣üÓĖŻÓĖćÓĖĪÓĖ▓ÓĖüÓĖéÓĖČÓ╣ēÓĖÖ Ó╣ł ÓĖäÓĖ¦ÓĖ▓ÓĖĪÓĖóÓĖ▓ÓĖ¦ÓĖ×ÓĖ▒ÓĖÖÓĖśÓĖ░ ÓĖĪÓĖĖÓĖĪÓĖ×ÓĖ▒ÓĖÖÓĖśÓĖ░ Ó╣ĆÓĖøÓĖźÓĖĄÓĖóÓĖÖÓ╣üÓĖøÓĖźÓĖć Ó╣ł Ó╣ĆÓĖøÓĖźÓĖĄÓĖóÓĖÖÓ╣üÓĖøÓĖźÓĖć Ó╣ł 21/11/54 Parinya T./2001 8
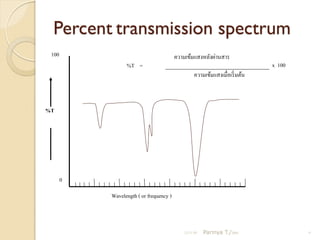
- 9. Percent transmission spectrum 100 ÓĖäÓĖ¦ÓĖ▓ÓĖĪÓ╣ĆÓĖéÓ╣ēÓĖĪÓ╣üÓĖ¬ÓĖćÓĖ½ÓĖźÓĖ▒ÓĖćÓĖ£Ó╣łÓĖ▓ÓĖÖÓĖ¬ÓĖ▓ÓĖŻ %T = x 100 ÓĖäÓĖ¦ÓĖ▓ÓĖĪÓ╣ĆÓĖéÓ╣ēÓĖĪÓ╣üÓĖ¬ÓĖćÓ╣ĆÓĖĪÓĖĘÓ╣łÓĖŁÓ╣ĆÓĖŻÓĖ┤Ó╣ł ÓĖĪÓĖĢÓ╣ēÓĖÖ %T 0 Wavelength ( or frequency ) 21/11/54 Parinya T./2001 9
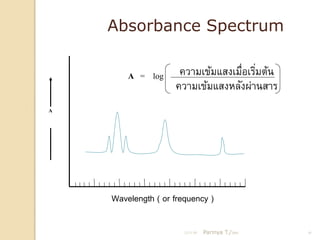
- 10. Absorbance Spectrum A = log ÓĖäÓĖ¦ÓĖ▓ÓĖĪÓ╣ĆÓĖéÓ╣ēÓĖĪÓ╣üÓĖ¬ÓĖćÓ╣ĆÓĖĪÓĖĘÓĖŁÓ╣ĆÓĖŻÓĖ┤ÓĖĪÓĖĢÓ╣ēÓĖÖ Ó╣ł Ó╣ł ÓĖäÓĖ¦ÓĖ▓ÓĖĪÓ╣ĆÓĖéÓ╣ēÓĖĪÓ╣üÓĖ¬ÓĖćÓĖ½ÓĖźÓĖ▒ÓĖćÓĖ£Ó╣łÓĖ▓ÓĖÖÓĖ¬ÓĖ▓ÓĖŻ A Wavelength ( or frequency ) 21/11/54 Parinya T./2001 10
- 11. ÓĖüÓĖ▓ÓĖŻÓĖöÓĖ╣ÓĖöÓĖüÓĖźÓĖĘÓĖÖÓĖäÓĖźÓĖĘÓ╣łÓĖÖÓĖŻÓĖ▒ÓĖćÓĖ¬ÓĖĄÓĖäÓĖ¦ÓĖ▓ÓĖĪÓĖŻÓ╣ē ÓĖŁÓĖÖ ÓĖ×ÓĖ▒ÓĖÖÓĖśÓĖ░Ó╣éÓĖäÓ╣ĆÓĖ¦Ó╣ĆÓĖźÓĖÖÓĖŚÓ╣īÓ╣ĆÓĖŖÓĖĘÓ╣łÓĖŁÓĖĪÓĖÖÓĖ┤ÓĖ¦Ó╣ĆÓĖäÓĖźÓĖĄÓĖóÓĖ¬ÓĖéÓĖŁÓĖćÓĖŁÓĖ░ÓĖĢÓĖŁÓĖĪ 2 ÓĖŁÓĖ░ÓĖĢÓĖŁÓĖĪÓ╣ĆÓĖéÓ╣ēÓĖ▓Ó╣äÓĖ¦Ó╣ē ÓĖäÓĖźÓ╣ēÓĖ▓ÓĖóÓĖ¬ÓĖøÓĖŻÓĖ┤ÓĖćÓĖóÓĖČÓĖöÓĖ¦ÓĖ▒ÓĖĢÓĖ¢ÓĖĖ 2 ÓĖŖÓĖ┤ÓĖÖÓ╣äÓĖ¦Ó╣ēÓĖöÓĖ¦ÓĖóÓĖüÓĖ▒ÓĖÖ ÓĖäÓĖŻÓĖ▒ÓĖÖÓ╣ĆÓĖĪÓĖĘÓĖŁÓ╣äÓĖöÓ╣ēÓĖŻÓĖÜÓĖ×ÓĖźÓĖ▒ÓĖćÓĖćÓĖ▓ÓĖÖÓĖłÓĖ▓ÓĖü Ó╣ē Ó╣ē Ó╣ē Ó╣ł ÓĖ▒ ÓĖäÓĖźÓĖĘÓ╣łÓĖÖÓĖŻÓĖ▒ÓĖćÓĖ¬ÓĖĄÓĖäÓĖ¦ÓĖ▓ÓĖĪÓĖŻÓ╣ēÓĖŁÓĖÖÓĖŚÓĖĄÓĖĪÓĖäÓĖ¦ÓĖ▓ÓĖĪÓĖ¢ÓĖĄÓ╣łÓĖ×ÓĖŁÓ╣ĆÓĖ½ÓĖĪÓĖ▓ÓĖ░ ÓĖüÓ╣ćÓĖłÓĖ░ÓĖ¬ÓĖ▒ÓĖÖÓ╣üÓĖŻÓĖćÓĖéÓĖČÓĖÖÓĖłÓĖ▓ÓĖüÓĖøÓĖüÓĖĢÓĖ┤ Ó╣ł ÓĖĄ Ó╣ł Ó╣ē Ó╣ĆÓĖŻÓĖĄÓĖóÓĖüÓĖ¦Ó╣łÓĖ▓Ó╣éÓĖĪÓ╣ĆÓĖźÓĖüÓĖĖÓĖźÓĖÖÓĖĄÓ╣ēÓĖüÓĖ▓ÓĖźÓĖ▒ÓĖćÓĖŁÓĖóÓĖ╣Ó╣āÓĖÖ vibrational excited state Ó╣ł Fact 1. ÓĖ×ÓĖ▒ÓĖÖÓĖśÓĖ░ÓĖĢÓ╣łÓĖ▓ÓĖćÓĖŖÓĖÖÓĖ┤ÓĖöÓĖüÓĖ▒ÓĖÖÓĖłÓĖ░ÓĖöÓĖ╣ÓĖöÓĖüÓĖźÓĖĘÓĖÖÓĖŻÓĖ▒ÓĖćÓĖ¬ÓĖĄÓĖŁÓĖÖÓĖ¤ÓĖŻÓĖ▓Ó╣ĆÓĖŻÓĖöÓĖŚÓĖĄÓĖĪÓĖäÓĖ¦ÓĖ▓ÓĖĪÓĖ¢ÓĖĄÓĖĢÓ╣łÓĖ▓ÓĖćÓĖüÓĖ▒ÓĖÖ ÓĖ┤ Ó╣ł ÓĖĄ Ó╣ł 11
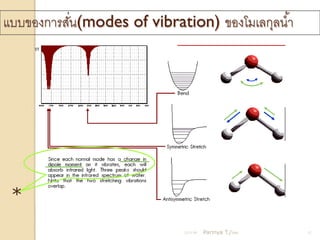
- 12. Ó╣üÓĖÜÓĖÜÓĖéÓĖŁÓĖćÓĖüÓĖ▓ÓĖŻÓĖ¬ÓĖ▒ÓĖÖ(modes of vibration) ÓĖéÓĖŁÓĖćÓ╣éÓĖĪÓ╣ĆÓĖźÓĖüÓĖĖÓĖźÓĖÖ Ó╣ēÓĖ▓ Ó╣ł * 21/11/54 Parinya T./2001 12
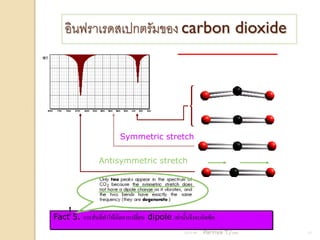
- 13. ÓĖŁÓĖ┤ÓĖÖÓĖ¤ÓĖŻÓĖ▓Ó╣ĆÓĖŻÓĖöÓĖ¬Ó╣ĆÓĖøÓĖüÓĖĢÓĖŻÓĖ▒ÓĖĪÓĖéÓĖŁÓĖć carbon dioxide bend Symmetric stretch Antisymmetric stretch ! Fact 5. ÓĖüÓĖ▓ÓĖŻÓĖ¬ÓĖ▒Ó╣łÓĖÖÓĖŚÓĖĄÓ╣łÓĖŚÓĖ▓Ó╣āÓĖ½Ó╣ēÓ╣ĆÓĖüÓĖ┤ÓĖöÓĖüÓĖ▓ÓĖŻÓ╣ĆÓĖøÓĖźÓĖĄÓ╣łÓĖóÓĖÖ dipole Ó╣ĆÓĖŚÓ╣łÓĖ▓ÓĖÖÓĖ▒Ó╣ēÓĖÖÓĖłÓĖČÓĖćÓĖłÓĖ░Ó╣ĆÓĖüÓĖ┤ÓĖöÓĖ×ÓĖĄÓĖä 21/11/54 Parinya T./2001 13
- 14. INFRARED SPECTROSCOPY ’āś Infrared radiation stimulates molecular vibrations. ’āś Infrared spectra are traditionally displayed as %T (percent transmittance) versus wavenumber (4000-400 cm-1). ’āś Useful in identifying presence or absence of functional groups.
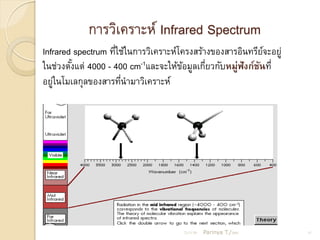
- 15. ÓĖüÓĖ▓ÓĖŻÓĖ¦ÓĖ┤Ó╣ĆÓĖäÓĖŻÓĖ▓ÓĖ░ÓĖ½Ó╣ī Infrared Spectrum Infrared spectrum ÓĖŚÓĖĄÓ╣āÓĖŖÓ╣ēÓ╣āÓĖÖÓĖüÓĖ▓ÓĖŻÓĖ¦ÓĖ┤Ó╣ĆÓĖäÓĖŻÓĖ▓ÓĖ░ÓĖ½Ó╣īÓ╣éÓĖäÓĖŻÓĖćÓĖ¬ÓĖŻÓ╣ēÓĖ▓ÓĖćÓĖéÓĖŁÓĖćÓĖ¬ÓĖ▓ÓĖŻÓĖŁÓĖ┤ÓĖÖÓĖŚÓĖŻÓĖĄÓĖóÓĖłÓĖ░ÓĖŁÓĖóÓĖ╣Ó╣ł Ó╣ł Ó╣ī Ó╣āÓĖÖÓĖŖÓ╣łÓĖ¦ÓĖćÓĖĢÓĖ▒ÓĖćÓ╣üÓĖĢÓ╣ł 4000 - 400 cm-1Ó╣üÓĖźÓĖ░ÓĖłÓĖ░Ó╣āÓĖ½Ó╣ēÓĖéÓĖŁÓĖĪÓĖ╣ÓĖźÓ╣ĆÓĖüÓĖĄÓĖóÓĖ¦ÓĖüÓĖ▒ÓĖÜÓĖ½ÓĖĪÓĖ╣Ó╣łÓĖ¤ÓĖ▒ÓĖćÓĖüÓ╣īÓĖŖÓĖÖÓĖŚÓĖĄÓ╣ł Ó╣ē Ó╣ē Ó╣ł ÓĖ▒ ÓĖŁÓĖóÓĖ╣Ó╣āÓĖÖÓ╣éÓĖĪÓ╣ĆÓĖźÓĖüÓĖĖÓĖźÓĖéÓĖŁÓĖćÓĖ¬ÓĖ▓ÓĖŻÓĖŚÓĖĄÓĖÖÓĖ▓ÓĖĪÓĖ▓ÓĖ¦ÓĖ┤Ó╣ĆÓĖäÓĖŻÓĖ▓ÓĖ░ÓĖ½Ó╣ī Ó╣ł Ó╣ł 21/11/54 Parinya T./2001 15
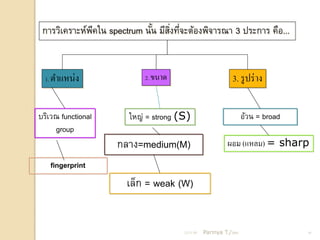
- 16. ÓĖüÓĖ▓ÓĖŻÓĖ¦ÓĖ┤Ó╣ĆÓĖäÓĖŻÓĖ▓ÓĖ░ÓĖ½Ó╣īÓĖ×ÓĖäÓ╣āÓĖÖ spectrum ÓĖÖÓĖ▒ÓĖÖ ÓĖĪÓĖĄÓĖ¬ÓĖćÓĖŚÓĖĄÓĖłÓĖ░ÓĖĢÓ╣ēÓĖŁÓĖćÓĖ×ÓĖ┤ÓĖłÓĖ▓ÓĖŻÓĖōÓĖ▓ 3 ÓĖøÓĖŻÓĖ░ÓĖüÓĖ▓ÓĖŻ ÓĖäÓĖĘÓĖŁ... ÓĖĄ Ó╣ē ÓĖ┤Ó╣ł Ó╣ł 1. ÓĖĢÓĖ▓Ó╣üÓĖ½ÓĖÖÓ╣łÓĖć 2. ÓĖéÓĖÖÓĖ▓ÓĖö 3. ÓĖŻÓĖ╣ ÓĖøÓĖŻÓ╣ł ÓĖ▓ÓĖć ÓĖÜÓĖŻÓĖ┤Ó╣ĆÓĖ¦ÓĖō functional Ó╣āÓĖ½ÓĖŹÓ╣ł = strong (S) ÓĖŁÓ╣ēÓĖ¦ÓĖÖ = broad group ÓĖüÓĖźÓĖ▓ÓĖć=medium(M) ÓĖ£ÓĖŁÓĖĪ (Ó╣üÓĖ½ÓĖźÓĖĪ) = sharp fingerprint Ó╣ĆÓĖźÓ╣ćÓĖü = weak (W) 21/11/54 Parinya T./2001 16
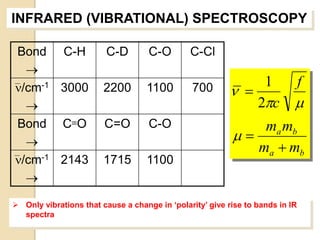
- 17. INFRARED (VIBRATIONAL) SPECTROSCOPY Bond C-H C-D C-O C-Cl ’é« ’ü«/cm-1 3000 1 f 2200 1100 700 ’ü« ’ĆĮ ’é« 2’ü░c ’üŁ Bond C’é║O C=O C-O ma mb ’é« ’üŁ’ĆĮ ma ’Ć½ mb ’ü«/cm-1 2143 1715 1100 ’é« ’āś Only vibrations that cause a change in ŌĆśpolarityŌĆÖ give rise to bands in IR spectra
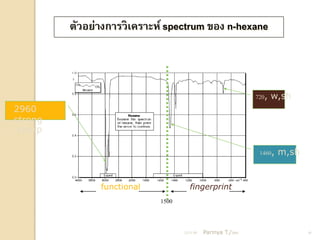
- 18. ÓĖĢÓĖ▒ÓĖ¦ÓĖŁÓĖóÓ╣łÓĖ▓ÓĖćÓĖüÓĖ▓ÓĖŻÓĖ¦ÓĖ┤Ó╣ĆÓĖäÓĖŻÓĖ▓ÓĖ░ÓĖ½Ó╣ī spectrum ÓĖéÓĖŁÓĖć n-hexane 720, w,sh 2960 strong ,sharp 1460, m,sh functional fingerprint 1500 21/11/54 Parinya T./2001 18
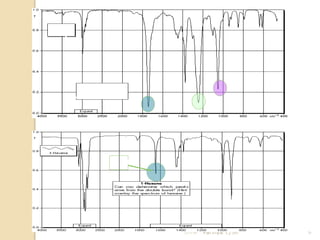
- 19. ÓĖŖÓ╣łÓĖ¦ÓĖć fingerprint Ó╣üÓĖĢÓĖüÓĖĢÓ╣ł ÓĖ▓ÓĖćÓĖüÓĖ▒ÓĖÖÓĖŖÓĖ▒ÓĖöÓ╣ĆÓĖłÓĖÖ 21/11/54 Parinya T./2001 19
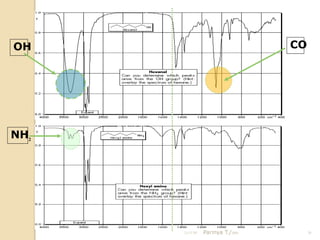
- 20. OH CO NH2 21/11/54 Parinya T./2001 20
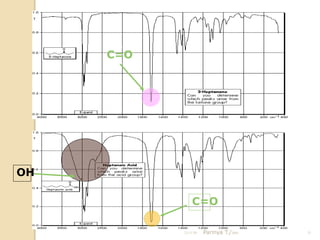
- 21. C=O OH C=O 21/11/54 Parinya T./2001 21
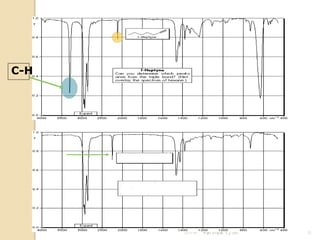
- 22. C-H 21/11/54 Parinya T./2001 22
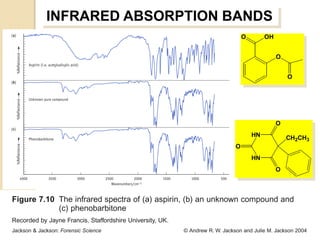
- 23. INFRARED ABSORPTION BANDS O OH O O O HN CH2CH3 O HN O
- 24. C=C 21/11/54 Parinya T./2001 24
- 25. INFRARED SPECTRA Acetone 120 100 80 O %T 60 C 40 H3C CH3 20 0 4000 3500 3000 2500 2000 1500 1000 500 wavenumber/ cm-1 Acetophenone 120 100 O 80 C %T 60 H3C 40 20 0 4000 3500 3000 2500 2000 1500 1000 500 wavenumber/ cm-1
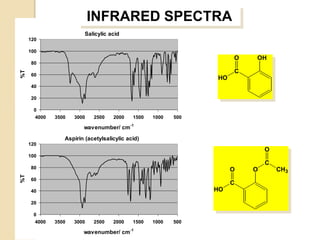
- 26. INFRARED SPECTRA Salicylic acid 120 100 O OH 80 C %T 60 HO 40 20 0 4000 3500 3000 2500 2000 1500 1000 500 wavenumber/ cm-1 Aspirin (acetylsalicylic acid) 120 O 100 C 80 O O CH3 %T 60 C 40 HO 20 0 4000 3500 3000 2500 2000 1500 1000 500 wavenumber/ cm-1
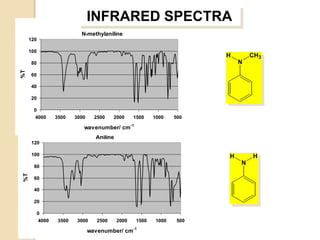
- 27. INFRARED SPECTRA N-methylaniline 120 100 H CH3 80 N %T 60 40 20 0 4000 3500 3000 2500 2000 1500 1000 500 wavenumber/ cm-1 Aniline 120 100 H H N 80 %T 60 40 20 0 4000 3500 3000 2500 2000 1500 1000 500 wavenumber/ cm-1
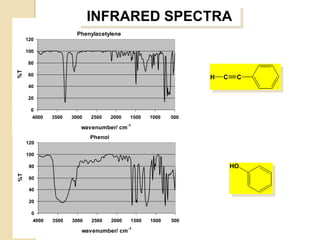
- 28. INFRARED SPECTRA Phenylacetylene 120 100 80 %T 60 H C C 40 20 0 4000 3500 3000 2500 2000 1500 1000 500 wavenumber/ cm-1 Phenol 120 100 80 HO %T 60 40 20 0 4000 3500 3000 2500 2000 1500 1000 500 wavenumber/ cm-1