Biosafety-Guidelines & Levels basics.pptx
Download as PPTX, PDF0 likes38 views
you can know about the basic information about the biosafety levels and guideliness
1 of 16
Download to read offline















Recommended
BRM Training Manual



BRM Training ManualAshfaq Ahmad
╠²
This training manual provides guidance on biorisk management for laboratory workers, field personnel, and research students working in veterinary laboratories in Pakistan. Biorisk management is important to control safety and security risks associated with handling biological materials and prevent unintentional exposure and accidental release. The manual covers terminology related to biorisk management, the scope and importance of establishing biorisk management systems in facilities, and the objectives of providing biorisk management training to raise awareness of biosafety and biosecurity practices.Bio-Safety Guidelines



Bio-Safety GuidelinesShahbaz Ahmad
╠²
The document provides a detailed overview on the basic principles of operating a biotech or micro laboratory along with basic techniques with which to handle organisms, chemicals &equipment and ensuring your own, your colleagues and your environment's safety.Biosafety & biosecurity lab biosecurity



Biosafety & biosecurity lab biosecurityAsif nawaz khan (AUST)
╠²
The Laboratory biosafety emphasizes the use of good microbiological practices, appropriate containment equipment, proper facility design, operation/maintenance and administrative considerations to minimize the risk of worker injury or illness.
BIOSAFETY AND INDIAN ETHICS (DRA)



BIOSAFETY AND INDIAN ETHICS (DRA)APCER Life Sciences
╠²
Biosafety╠²is the prevention of large-scale loss of biological integrity, focusing both on ecology and human health. These prevention mechanisms include conduction of regular reviews of the biosafety╠²in laboratory settings, as well as strict guidelines to follow. Biosafety also means safety from exposure to infectious agents.
Necessity
In order to avoid infection/biohazard to the laboratory personnel & the environment, biosafety levels are very important.Biosafety and biocontainment concepts and strategies.pptx



Biosafety and biocontainment concepts and strategies.pptxridazaynab01
╠²
biosafety and biocontainment; concepts and strategies
Zoology | Biorisk | Biosafety | Biocontainment | Lab practices 10 safety guidelines for recombinant dna research



10 safety guidelines for recombinant dna researchIndranil Bhattacharjee
╠²
This document discusses biosafety guidelines for recombinant DNA research. It defines biosafety as applying safety principles to potentially hazardous biological materials or organisms. Guidelines have been developed by organizations like the National Institutes of Health and Department of Biotechnology in India. There are four biosafety levels depending on the risk posed by the organisms and experiments, with increasing safety requirements at higher levels. Risk assessment involves evaluating characteristics of the organisms and modifications to determine the biosafety level needed. Risk management aims to minimize risks to human health and the environment through prevention measures and policies.Safety considerations and guidelines veterinary microbiology laboratory



Safety considerations and guidelines veterinary microbiology laboratoryRavi Kant Agrawal
╠²
This document provides guidelines on biosafety and biosecurity for veterinary microbiology laboratories. It defines key terms like biohazard, biosafety, risk assessment, biosecurity, and the biohazard symbol. It discusses the chain of infection and approaches to reduce risk of exposure like risk assessment, personal protective equipment, immunizations, and surveillance. The document also compares and contrasts biosecurity and biosafety. It provides guidance on developing a biosecurity program and addressing breaches. It discusses challenges of preventing interference while ensuring legitimate access.Biosafety lecture 1[1]![Biosafety lecture 1[1]](https://cdn.slidesharecdn.com/ss_thumbnails/biosafetylecture11-180117235846-thumbnail.jpg?width=560&fit=bounds)
![Biosafety lecture 1[1]](https://cdn.slidesharecdn.com/ss_thumbnails/biosafetylecture11-180117235846-thumbnail.jpg?width=560&fit=bounds)
![Biosafety lecture 1[1]](https://cdn.slidesharecdn.com/ss_thumbnails/biosafetylecture11-180117235846-thumbnail.jpg?width=560&fit=bounds)
![Biosafety lecture 1[1]](https://cdn.slidesharecdn.com/ss_thumbnails/biosafetylecture11-180117235846-thumbnail.jpg?width=560&fit=bounds)
Biosafety lecture 1[1]Jamilu Yarima
╠²
This document discusses biosafety principles for microbiology and biomedical laboratories. It defines biosafety and outlines key concepts like biohazards, biosafety levels, and the biohazard symbol. Biosafety aims to minimize health and environmental risks from hazardous biological materials through administrative controls, safety equipment, and facility design tailored to the risks involved. The document also notes emerging issues at the intersection of biosafety and biotechnology like genetically modified organisms, biosecurity, and bioethics.Laboratory biosafety levels and transfer of biological samples



Laboratory biosafety levels and transfer of biological samplesMintah Dadzie Francis
╠²
This document discusses laboratory biosafety levels and the transfer of biological samples. It begins by outlining the objectives and presentation outline. It then introduces biosafety practices and discusses the four biosafety levels - BSL-1 to BSL-4 - in increasing order of risk. Each level is associated with different safety practices and containment facilities depending on the risk of pathogens handled. The document also discusses regulations for the safe transfer of biological samples between facilities, including packaging, labeling and documentation requirements. Material transfer agreements are identified as important for governing the transfer and use of biological materials.David Glass BIO World Congress Synthetic Biology Regulation july 2015



David Glass BIO World Congress Synthetic Biology Regulation july 2015David Glass
╠²
Presentation from July 2015 BIO World Congress on Industrial Biotechnology, assessing the adequacy of government regulatory frameworks to assess the risks of commercial uses of synthetic biology.Tamu Fall From Grace



Tamu Fall From GraceMalcolm Smith
╠²
This document provides an overview of events related to exposures to select agents at Texas A&M University's biodefense research laboratories. It discusses the characteristics of select agents and regulations governing their use. It describes safety protocols for BSL-3 laboratories and summarizes inspections that found issues with Texas A&M's brucellosis and Coxiella research resulting in researcher exposures. The document also provides background on the university and its biodefense research programs.Amee write-up on - POTENTIAL HAZARDS ASSOCIATED WITH WORK PRACTICES



Amee write-up on - POTENTIAL HAZARDS ASSOCIATED WITH WORK PRACTICESamee terdue
╠²
This document summarizes a workshop paper on potential biohazards in a pathological department associated with daily work practices. It discusses hazards from work practices, safety equipment, and facility safeguards. Potential hazards include exposure to microbial aerosols and droplets from improperly functioning biosafety cabinets, sealed rotors, or ventilation systems. Training and maintenance of safety equipment is important to ensure proper operation and containment. Facility safeguards like directional airflow also help prevent accidental release of agents and their transmission to other areas. A risk assessment approach is outlined to identify agent and procedure hazards, determine the appropriate biosafety level, and select additional precautions to reduce risks to workers.Amee write-up on - POTENTIAL HAZARDS ASSOCIATED WITH WORK PRACTICES



Amee write-up on - POTENTIAL HAZARDS ASSOCIATED WITH WORK PRACTICESamee terdue
╠²
This document summarizes the potential bio-hazards associated with daily work practices in a pathological department. It discusses hazards from work practices, safety equipment, and facility safeguards. Proper training and safety protocols are important to reduce risks of exposure to hazardous agents. A risk assessment should evaluate hazards from agents, laboratory procedures, equipment functionality, and staff proficiency. Reviews with safety experts can help ensure appropriate safety levels.04 control of spills and mechanism of implementation of biosafety guidelines



04 control of spills and mechanism of implementation of biosafety guidelinesIndranil Bhattacharjee
╠²
1. The document outlines procedures for controlling spills of biological materials in laboratories, including spills in biological safety cabinets, open laboratories, centrifuges, and on persons. It describes wearing protective equipment, warning others, and using appropriate disinfectants.
2. It then describes the mechanism for implementing biosafety guidelines in India, including committees that provide oversight of recombinant DNA research. The key committees are the Recombinant DNA Advisory Committee, Institutional Biosafety Committees, and the Review Committee on Genetic Manipulation.
3. The committees provide guidance on biosafety, review research proposals, oversee safety training, and ensure containment facilities and procedures are followed to regulate genetic engineering activities and protectTi├¬u chuß║®n ph├▓ng sß║Īch trong sß║Żn xuß║źt vaccine 



Ti├¬u chuß║®n ph├▓ng sß║Īch trong sß║Żn xuß║źt vaccine C├┤ng ty Cß╗Ģ phß║¦n TŲ░ vß║źn Thiß║┐t kß║┐ GMP EU
╠²
Ti├¬u chuß║®n ph├▓ng sß║Īch trong sß║Żn xuß║źt vaccine . Xem th├¬m c├Īc t├Āi liß╗ću kh├Īc tr├¬n k├¬nh cß╗¦a C├┤ng ty Cß╗Ģ phß║¦n TŲ░ vß║źn Thiß║┐t kß║┐ GMP EUWorkshop report40



Workshop report40Chandra Shekar
╠²
1) The document discusses the history and definitions of biomarkers from the 1960s to 1990s based on a literature review. It shows a progression from early uses related to diagnosing cancer to later applications in toxicology.
2) By the 1980s, biomarkers were being used to study occupational exposure and effects of toxic substances. Studies also began exploring their potential role in risk assessment.
3) In the 1990s, biomarkers were applied to monitoring the effects of interventions aimed at reducing disease risk by modifying exposure to suspected causal agents. This supported establishing exposure-disease relationships.Biosafety level



Biosafety levelshivanki verma
╠²
This document presents guidelines on biosafety from the Government of India. It discusses the history and necessity of biosafety, describing the four biosafety levels established by the Centers for Disease Control and Prevention for handling infectious agents. It also outlines the roles of the Institutional Biosafety Committee, which reviews research using hazardous organisms, and the Genetic Engineering Appraisal Committee, which approves large-scale production of genetically modified organisms. The document emphasizes the importance of containment methods like physical barriers and biological techniques to prevent the spread of microbes in laboratories.Biosafety in the Realm of DURC Part 1.pptx



Biosafety in the Realm of DURC Part 1.pptxwmcrcrt8w8
╠²
Biosafety issues in dual-use infectious disease research involving microorganisms.Cdc guideline for disinfection and sterilization in healthcare facilities, 2008



Cdc guideline for disinfection and sterilization in healthcare facilities, 2008Enrique Guillen
╠²
This document provides guidelines for disinfection and sterilization in healthcare facilities. It discusses various methods of cleaning, disinfection and sterilization for patient care equipment. It categorizes items based on risk of infection as critical, semi-critical or non-critical and provides recommendations for processes to reduce bioburden for each category. New topics covered include inactivation of antibiotic resistant and emerging pathogens, toxicological concerns, and disinfection of equipment used in ambulatory and home care settings. The guidelines are intended to help standardize practices and reduce infection risks when using medical devices and surgical instruments.Guideline for disinfection and sterilization in healthcare facilities, 2008[1]![Guideline for disinfection and sterilization in healthcare facilities, 2008[1]](https://cdn.slidesharecdn.com/ss_thumbnails/guidelinefordisinfectionandsterilizationinhealthcarefacilities20081-101114110924-phpapp01-thumbnail.jpg?width=560&fit=bounds)
![Guideline for disinfection and sterilization in healthcare facilities, 2008[1]](https://cdn.slidesharecdn.com/ss_thumbnails/guidelinefordisinfectionandsterilizationinhealthcarefacilities20081-101114110924-phpapp01-thumbnail.jpg?width=560&fit=bounds)
![Guideline for disinfection and sterilization in healthcare facilities, 2008[1]](https://cdn.slidesharecdn.com/ss_thumbnails/guidelinefordisinfectionandsterilizationinhealthcarefacilities20081-101114110924-phpapp01-thumbnail.jpg?width=560&fit=bounds)
![Guideline for disinfection and sterilization in healthcare facilities, 2008[1]](https://cdn.slidesharecdn.com/ss_thumbnails/guidelinefordisinfectionandsterilizationinhealthcarefacilities20081-101114110924-phpapp01-thumbnail.jpg?width=560&fit=bounds)
Guideline for disinfection and sterilization in healthcare facilities, 2008[1]Manel Ferreira
╠²
This document provides guidelines for disinfection and sterilization in healthcare facilities. It discusses various methods of cleaning, disinfection and sterilization for patient care equipment. It categorizes items based on risk of infection as critical, semi-critical or non-critical and provides recommendations for processes to reduce risk of infection from each category. New topics covered include inactivation of antibiotic resistant and emerging pathogens, toxicological concerns, and disinfection of equipment used in ambulatory and home care settings.NJ Hazardous Drug Safe Handling Act



NJ Hazardous Drug Safe Handling ActThe Windsdor Consulting Group, Inc.
╠²
Under the former NJ Governor Christie administration, a 2017 law was passed to begin the process of identifying stakeholders who may be able to construct legislation to protect healthcare workers who are exposed to hazardous drugs in oncology. Some work practices place pharmacists, animal handlers, veterinarians, oncologists and nurses at risk of contact with these toxic drugs. Many of these drugs cause terratogenic and mutagenic effects in both men, women, and offspring. The National Institute for Occupational Safety and Health (NIOSH) identified 204 hazardous drugs, which may be toxic to exposed workers. In addition, both the State of Washington and California have enacted legislation to protect all workers from exposure, which includes training, biological monitoring and medical surveillance. This is a very important issue that needs further review and consideration.Biosafety and waste management in histopathology



Biosafety and waste management in histopathologyJennifer Giovanna
╠²
The document discusses biosafety and waste management in histopathology labs. It outlines biosafety level guidelines which classify medical labs and microorganisms into four levels based on architectural features, ventilation, and safety equipment. It describes the basic lab and containment lab designs and safety practices like limited access, decontamination, and personal protective equipment. It also categorizes pathological waste, discusses principles of effective waste management including segregation, collection, storage, transportation, and treatment, and provides recommendations to improve biosafety standards.Viral Risk Mitigation - A Global Regulatory Perspective



Viral Risk Mitigation - A Global Regulatory PerspectiveMilliporeSigma
╠²
Looking for insights into current global regulatory expectations for viral safety? Read the special report from BioProcess International, in collaboration with Martin Wisher, Senior Regulatory Consultant focusing on BioReliance biosafety® services.Viral Risk Mitigation - A Global Regulatory Perspective



Viral Risk Mitigation - A Global Regulatory PerspectiveMerck Life Sciences
╠²
This document provides an overview of global regulatory guidance for ensuring viral safety in biologics production. It discusses three key approaches: preventing contamination through high quality raw materials; detecting contamination through testing cell banks, raw materials, and process intermediates; and evaluating viral clearance in the production process. The summary discusses the types of regulatory documents that provide guidelines on raw materials and cell lines, as well as strategies for preventing contamination, detecting contamination through a variety of assay methods, and limitations of detection assays.Clinical lab principles, chapter 2 introduction to principles of lab analyses...



Clinical lab principles, chapter 2 introduction to principles of lab analyses...Ali Raza Ph.D
╠²
This document provides an introduction to laboratory safety principles including safety programs, policies, plans, hazards, and precautions. It discusses establishing a formal safety program with documented policies on chemical hygiene, exposure control, tuberculosis control and ergonomics. The major occupational hazards of biological agents, chemicals, fires, electricity and compressed gases are outlined. Precautions for working safely include use of personal protective equipment, proper chemical handling and labeling, and avoiding mouth pipetting.CDC - After-Action Report Anthrax Incident



CDC - After-Action Report Anthrax IncidentDawn Dawson
╠²
CDC Director Releases After-Action Report on Recent Anthrax Incident; Highlights Steps to Improve Laboratory Quality and Safety http://www.cdc.gov/od/science/integrity/docs/Final_Anthrax_Report.pdf GLP 21 CFR part 58



GLP 21 CFR part 58Dr.K.Venkateswara raju
╠²
This document discusses Good Laboratory Practices (GLP), which are regulations created by the FDA in 1978 to ensure quality and integrity in nonclinical laboratory studies. It establishes standards for laboratory organization and management, personnel, facilities, equipment, testing operations, and recordkeeping. Key aspects include requiring standard operating procedures, designated study directors, quality assurance units to conduct inspections, maintaining facilities and equipment, ensuring personnel qualifications, and properly housing, caring for, and identifying laboratory animals. GLP aims to eliminate fraudulent activities and poor practices identified in investigations of laboratories in the 1970s.An introduction on biosaftey



An introduction on biosafteyVipin Shukla
╠²
Biosaftey means the needs to protect human and animal health along with the environment from the possible adverse effects of the products of modern biotechnology. Biosafety defines the containment conditions under which infectious agents can be safely manipulated. Biosafety word is used to reduce and eliminate the potential risk regulating from the modern biotechnology and its products.Blind Spots in AI and Formulation Science Knowledge Pyramid (Updated Perspect...



Blind Spots in AI and Formulation Science Knowledge Pyramid (Updated Perspect...Ajaz Hussain
╠²
This presentation delves into the systemic blind spots within pharmaceutical science and regulatory systems, emphasizing the significance of "inactive ingredients" and their influence on therapeutic equivalence. These blind spots, indicative of normalized systemic failures, go beyond mere chance occurrences and are ingrained deeply enough to compromise decision-making processes and erode trust.
Historical instances like the 1938 FD&C Act and the Generic Drug Scandals underscore how crisis-triggered reforms often fail to address the fundamental issues, perpetuating inefficiencies and hazards.
The narrative advocates a shift from reactive crisis management to proactive, adaptable systems prioritizing continuous enhancement. Key hurdles involve challenging outdated assumptions regarding bioavailability, inadequately funded research ventures, and the impact of vague language in regulatory frameworks.
The rise of large language models (LLMs) presents promising solutions, albeit with accompanying risks necessitating thorough validation and seamless integration.
Tackling these blind spots demands a holistic approach, embracing adaptive learning and a steadfast commitment to self-improvement. By nurturing curiosity, refining regulatory terminology, and judiciously harnessing new technologies, the pharmaceutical sector can progress towards better public health service delivery and ensure the safety, efficacy, and real-world impact of drug products.More Related Content
Similar to Biosafety-Guidelines & Levels basics.pptx (20)
Laboratory biosafety levels and transfer of biological samples



Laboratory biosafety levels and transfer of biological samplesMintah Dadzie Francis
╠²
This document discusses laboratory biosafety levels and the transfer of biological samples. It begins by outlining the objectives and presentation outline. It then introduces biosafety practices and discusses the four biosafety levels - BSL-1 to BSL-4 - in increasing order of risk. Each level is associated with different safety practices and containment facilities depending on the risk of pathogens handled. The document also discusses regulations for the safe transfer of biological samples between facilities, including packaging, labeling and documentation requirements. Material transfer agreements are identified as important for governing the transfer and use of biological materials.David Glass BIO World Congress Synthetic Biology Regulation july 2015



David Glass BIO World Congress Synthetic Biology Regulation july 2015David Glass
╠²
Presentation from July 2015 BIO World Congress on Industrial Biotechnology, assessing the adequacy of government regulatory frameworks to assess the risks of commercial uses of synthetic biology.Tamu Fall From Grace



Tamu Fall From GraceMalcolm Smith
╠²
This document provides an overview of events related to exposures to select agents at Texas A&M University's biodefense research laboratories. It discusses the characteristics of select agents and regulations governing their use. It describes safety protocols for BSL-3 laboratories and summarizes inspections that found issues with Texas A&M's brucellosis and Coxiella research resulting in researcher exposures. The document also provides background on the university and its biodefense research programs.Amee write-up on - POTENTIAL HAZARDS ASSOCIATED WITH WORK PRACTICES



Amee write-up on - POTENTIAL HAZARDS ASSOCIATED WITH WORK PRACTICESamee terdue
╠²
This document summarizes a workshop paper on potential biohazards in a pathological department associated with daily work practices. It discusses hazards from work practices, safety equipment, and facility safeguards. Potential hazards include exposure to microbial aerosols and droplets from improperly functioning biosafety cabinets, sealed rotors, or ventilation systems. Training and maintenance of safety equipment is important to ensure proper operation and containment. Facility safeguards like directional airflow also help prevent accidental release of agents and their transmission to other areas. A risk assessment approach is outlined to identify agent and procedure hazards, determine the appropriate biosafety level, and select additional precautions to reduce risks to workers.Amee write-up on - POTENTIAL HAZARDS ASSOCIATED WITH WORK PRACTICES



Amee write-up on - POTENTIAL HAZARDS ASSOCIATED WITH WORK PRACTICESamee terdue
╠²
This document summarizes the potential bio-hazards associated with daily work practices in a pathological department. It discusses hazards from work practices, safety equipment, and facility safeguards. Proper training and safety protocols are important to reduce risks of exposure to hazardous agents. A risk assessment should evaluate hazards from agents, laboratory procedures, equipment functionality, and staff proficiency. Reviews with safety experts can help ensure appropriate safety levels.04 control of spills and mechanism of implementation of biosafety guidelines



04 control of spills and mechanism of implementation of biosafety guidelinesIndranil Bhattacharjee
╠²
1. The document outlines procedures for controlling spills of biological materials in laboratories, including spills in biological safety cabinets, open laboratories, centrifuges, and on persons. It describes wearing protective equipment, warning others, and using appropriate disinfectants.
2. It then describes the mechanism for implementing biosafety guidelines in India, including committees that provide oversight of recombinant DNA research. The key committees are the Recombinant DNA Advisory Committee, Institutional Biosafety Committees, and the Review Committee on Genetic Manipulation.
3. The committees provide guidance on biosafety, review research proposals, oversee safety training, and ensure containment facilities and procedures are followed to regulate genetic engineering activities and protectTi├¬u chuß║®n ph├▓ng sß║Īch trong sß║Żn xuß║źt vaccine 



Ti├¬u chuß║®n ph├▓ng sß║Īch trong sß║Żn xuß║źt vaccine C├┤ng ty Cß╗Ģ phß║¦n TŲ░ vß║źn Thiß║┐t kß║┐ GMP EU
╠²
Ti├¬u chuß║®n ph├▓ng sß║Īch trong sß║Żn xuß║źt vaccine . Xem th├¬m c├Īc t├Āi liß╗ću kh├Īc tr├¬n k├¬nh cß╗¦a C├┤ng ty Cß╗Ģ phß║¦n TŲ░ vß║źn Thiß║┐t kß║┐ GMP EUWorkshop report40



Workshop report40Chandra Shekar
╠²
1) The document discusses the history and definitions of biomarkers from the 1960s to 1990s based on a literature review. It shows a progression from early uses related to diagnosing cancer to later applications in toxicology.
2) By the 1980s, biomarkers were being used to study occupational exposure and effects of toxic substances. Studies also began exploring their potential role in risk assessment.
3) In the 1990s, biomarkers were applied to monitoring the effects of interventions aimed at reducing disease risk by modifying exposure to suspected causal agents. This supported establishing exposure-disease relationships.Biosafety level



Biosafety levelshivanki verma
╠²
This document presents guidelines on biosafety from the Government of India. It discusses the history and necessity of biosafety, describing the four biosafety levels established by the Centers for Disease Control and Prevention for handling infectious agents. It also outlines the roles of the Institutional Biosafety Committee, which reviews research using hazardous organisms, and the Genetic Engineering Appraisal Committee, which approves large-scale production of genetically modified organisms. The document emphasizes the importance of containment methods like physical barriers and biological techniques to prevent the spread of microbes in laboratories.Biosafety in the Realm of DURC Part 1.pptx



Biosafety in the Realm of DURC Part 1.pptxwmcrcrt8w8
╠²
Biosafety issues in dual-use infectious disease research involving microorganisms.Cdc guideline for disinfection and sterilization in healthcare facilities, 2008



Cdc guideline for disinfection and sterilization in healthcare facilities, 2008Enrique Guillen
╠²
This document provides guidelines for disinfection and sterilization in healthcare facilities. It discusses various methods of cleaning, disinfection and sterilization for patient care equipment. It categorizes items based on risk of infection as critical, semi-critical or non-critical and provides recommendations for processes to reduce bioburden for each category. New topics covered include inactivation of antibiotic resistant and emerging pathogens, toxicological concerns, and disinfection of equipment used in ambulatory and home care settings. The guidelines are intended to help standardize practices and reduce infection risks when using medical devices and surgical instruments.Guideline for disinfection and sterilization in healthcare facilities, 2008[1]![Guideline for disinfection and sterilization in healthcare facilities, 2008[1]](https://cdn.slidesharecdn.com/ss_thumbnails/guidelinefordisinfectionandsterilizationinhealthcarefacilities20081-101114110924-phpapp01-thumbnail.jpg?width=560&fit=bounds)
![Guideline for disinfection and sterilization in healthcare facilities, 2008[1]](https://cdn.slidesharecdn.com/ss_thumbnails/guidelinefordisinfectionandsterilizationinhealthcarefacilities20081-101114110924-phpapp01-thumbnail.jpg?width=560&fit=bounds)
![Guideline for disinfection and sterilization in healthcare facilities, 2008[1]](https://cdn.slidesharecdn.com/ss_thumbnails/guidelinefordisinfectionandsterilizationinhealthcarefacilities20081-101114110924-phpapp01-thumbnail.jpg?width=560&fit=bounds)
![Guideline for disinfection and sterilization in healthcare facilities, 2008[1]](https://cdn.slidesharecdn.com/ss_thumbnails/guidelinefordisinfectionandsterilizationinhealthcarefacilities20081-101114110924-phpapp01-thumbnail.jpg?width=560&fit=bounds)
Guideline for disinfection and sterilization in healthcare facilities, 2008[1]Manel Ferreira
╠²
This document provides guidelines for disinfection and sterilization in healthcare facilities. It discusses various methods of cleaning, disinfection and sterilization for patient care equipment. It categorizes items based on risk of infection as critical, semi-critical or non-critical and provides recommendations for processes to reduce risk of infection from each category. New topics covered include inactivation of antibiotic resistant and emerging pathogens, toxicological concerns, and disinfection of equipment used in ambulatory and home care settings.NJ Hazardous Drug Safe Handling Act



NJ Hazardous Drug Safe Handling ActThe Windsdor Consulting Group, Inc.
╠²
Under the former NJ Governor Christie administration, a 2017 law was passed to begin the process of identifying stakeholders who may be able to construct legislation to protect healthcare workers who are exposed to hazardous drugs in oncology. Some work practices place pharmacists, animal handlers, veterinarians, oncologists and nurses at risk of contact with these toxic drugs. Many of these drugs cause terratogenic and mutagenic effects in both men, women, and offspring. The National Institute for Occupational Safety and Health (NIOSH) identified 204 hazardous drugs, which may be toxic to exposed workers. In addition, both the State of Washington and California have enacted legislation to protect all workers from exposure, which includes training, biological monitoring and medical surveillance. This is a very important issue that needs further review and consideration.Biosafety and waste management in histopathology



Biosafety and waste management in histopathologyJennifer Giovanna
╠²
The document discusses biosafety and waste management in histopathology labs. It outlines biosafety level guidelines which classify medical labs and microorganisms into four levels based on architectural features, ventilation, and safety equipment. It describes the basic lab and containment lab designs and safety practices like limited access, decontamination, and personal protective equipment. It also categorizes pathological waste, discusses principles of effective waste management including segregation, collection, storage, transportation, and treatment, and provides recommendations to improve biosafety standards.Viral Risk Mitigation - A Global Regulatory Perspective



Viral Risk Mitigation - A Global Regulatory PerspectiveMilliporeSigma
╠²
Looking for insights into current global regulatory expectations for viral safety? Read the special report from BioProcess International, in collaboration with Martin Wisher, Senior Regulatory Consultant focusing on BioReliance biosafety® services.Viral Risk Mitigation - A Global Regulatory Perspective



Viral Risk Mitigation - A Global Regulatory PerspectiveMerck Life Sciences
╠²
This document provides an overview of global regulatory guidance for ensuring viral safety in biologics production. It discusses three key approaches: preventing contamination through high quality raw materials; detecting contamination through testing cell banks, raw materials, and process intermediates; and evaluating viral clearance in the production process. The summary discusses the types of regulatory documents that provide guidelines on raw materials and cell lines, as well as strategies for preventing contamination, detecting contamination through a variety of assay methods, and limitations of detection assays.Clinical lab principles, chapter 2 introduction to principles of lab analyses...



Clinical lab principles, chapter 2 introduction to principles of lab analyses...Ali Raza Ph.D
╠²
This document provides an introduction to laboratory safety principles including safety programs, policies, plans, hazards, and precautions. It discusses establishing a formal safety program with documented policies on chemical hygiene, exposure control, tuberculosis control and ergonomics. The major occupational hazards of biological agents, chemicals, fires, electricity and compressed gases are outlined. Precautions for working safely include use of personal protective equipment, proper chemical handling and labeling, and avoiding mouth pipetting.CDC - After-Action Report Anthrax Incident



CDC - After-Action Report Anthrax IncidentDawn Dawson
╠²
CDC Director Releases After-Action Report on Recent Anthrax Incident; Highlights Steps to Improve Laboratory Quality and Safety http://www.cdc.gov/od/science/integrity/docs/Final_Anthrax_Report.pdf GLP 21 CFR part 58



GLP 21 CFR part 58Dr.K.Venkateswara raju
╠²
This document discusses Good Laboratory Practices (GLP), which are regulations created by the FDA in 1978 to ensure quality and integrity in nonclinical laboratory studies. It establishes standards for laboratory organization and management, personnel, facilities, equipment, testing operations, and recordkeeping. Key aspects include requiring standard operating procedures, designated study directors, quality assurance units to conduct inspections, maintaining facilities and equipment, ensuring personnel qualifications, and properly housing, caring for, and identifying laboratory animals. GLP aims to eliminate fraudulent activities and poor practices identified in investigations of laboratories in the 1970s.An introduction on biosaftey



An introduction on biosafteyVipin Shukla
╠²
Biosaftey means the needs to protect human and animal health along with the environment from the possible adverse effects of the products of modern biotechnology. Biosafety defines the containment conditions under which infectious agents can be safely manipulated. Biosafety word is used to reduce and eliminate the potential risk regulating from the modern biotechnology and its products.04 control of spills and mechanism of implementation of biosafety guidelines



04 control of spills and mechanism of implementation of biosafety guidelinesIndranil Bhattacharjee
╠²
Ti├¬u chuß║®n ph├▓ng sß║Īch trong sß║Żn xuß║źt vaccine 



Ti├¬u chuß║®n ph├▓ng sß║Īch trong sß║Żn xuß║źt vaccine C├┤ng ty Cß╗Ģ phß║¦n TŲ░ vß║źn Thiß║┐t kß║┐ GMP EU
╠²
Recently uploaded (20)
Blind Spots in AI and Formulation Science Knowledge Pyramid (Updated Perspect...



Blind Spots in AI and Formulation Science Knowledge Pyramid (Updated Perspect...Ajaz Hussain
╠²
This presentation delves into the systemic blind spots within pharmaceutical science and regulatory systems, emphasizing the significance of "inactive ingredients" and their influence on therapeutic equivalence. These blind spots, indicative of normalized systemic failures, go beyond mere chance occurrences and are ingrained deeply enough to compromise decision-making processes and erode trust.
Historical instances like the 1938 FD&C Act and the Generic Drug Scandals underscore how crisis-triggered reforms often fail to address the fundamental issues, perpetuating inefficiencies and hazards.
The narrative advocates a shift from reactive crisis management to proactive, adaptable systems prioritizing continuous enhancement. Key hurdles involve challenging outdated assumptions regarding bioavailability, inadequately funded research ventures, and the impact of vague language in regulatory frameworks.
The rise of large language models (LLMs) presents promising solutions, albeit with accompanying risks necessitating thorough validation and seamless integration.
Tackling these blind spots demands a holistic approach, embracing adaptive learning and a steadfast commitment to self-improvement. By nurturing curiosity, refining regulatory terminology, and judiciously harnessing new technologies, the pharmaceutical sector can progress towards better public health service delivery and ensure the safety, efficacy, and real-world impact of drug products.Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
╠²
Prelims of Rass MELAI : a Music, Entertainment, Literature, Arts and Internet Culture Quiz organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. How to Manage Putaway Rule in Odoo 17 Inventory



How to Manage Putaway Rule in Odoo 17 InventoryCeline George
╠²
Inventory management is a critical aspect of any business involved in manufacturing or selling products.
Odoo 17 offers a robust inventory management system that can handle complex operations and optimize warehouse efficiency. How to Configure Flexible Working Schedule in Odoo 18 Employee



How to Configure Flexible Working Schedule in Odoo 18 EmployeeCeline George
╠²
In this slide, weŌĆÖll discuss on how to configure flexible working schedule in Odoo 18 Employee module. In Odoo 18, the Employee module offers powerful tools to configure and manage flexible working schedules tailored to your organization's needs.The Story Behind the Abney Park Restoration Project by Tom Walker



The Story Behind the Abney Park Restoration Project by Tom WalkerHistory of Stoke Newington
╠²
Presented at the 24th Stoke Newington History Talks event on 27th Feb 2025
https://stokenewingtonhistory.com/stoke-newington-history-talks/Reordering Rules in Odoo 17 Inventory - Odoo ║▌║▌▀Żs



Reordering Rules in Odoo 17 Inventory - Odoo ║▌║▌▀ŻsCeline George
╠²
In Odoo 17, the Inventory module allows us to set up reordering rules to ensure that our stock levels are maintained, preventing stockouts. Let's explore how this feature works.Kaun TALHA quiz Prelims - El Dorado 2025



Kaun TALHA quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
╠²
Prelims of Kaun TALHA : a Travel, Architecture, Lifestyle, Heritage and Activism quiz, organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. TLE 7 - 3rd Topic - Hand Tools, Power Tools, Instruments, and Equipment Used ...



TLE 7 - 3rd Topic - Hand Tools, Power Tools, Instruments, and Equipment Used ...RizaBedayo
╠²
Hand Tools, Power Tools, and Equipment in Industrial ArtsEDL 290F Week 3 - Mountaintop Views (2025).pdf



EDL 290F Week 3 - Mountaintop Views (2025).pdfLiz Walsh-Trevino
╠²
EDL 290F Week 3 - Mountaintop Views (2025).pdfMate, a short story by Kate Grenville.pptx



Mate, a short story by Kate Grenville.pptxLiny Jenifer
╠²
A powerpoint presentation on the short story Mate by Kate Greenville. This presentation provides information on Kate Greenville, a character list, plot summary and critical analysis of the short story.Year 10 The Senior Phase Session 3 Term 1.pptx



Year 10 The Senior Phase Session 3 Term 1.pptxmansk2
╠²
Year 10 The Senior Phase Session 3 Term 1.pptxEssentials of a Good PMO, presented by Aalok Sonawala



Essentials of a Good PMO, presented by Aalok SonawalaAssociation for Project Management
╠²
APM event hosted by the South Wales and West of England Network (SWWE Network)
Speaker: Aalok Sonawala
The SWWE Regional Network were very pleased to welcome Aalok Sonawala, Head of PMO, National Programmes, Rider Levett Bucknall on 26 February, to BAWA for our first face to face event of 2025. Aalok is a member of APMŌĆÖs Thames Valley Regional Network and also speaks to members of APMŌĆÖs PMO Interest Network, which aims to facilitate collaboration and learning, offer unbiased advice and guidance.
Tonight, Aalok planned to discuss the importance of a PMO within project-based organisations, the different types of PMO and their key elements, PMO governance and centres of excellence.
PMOŌĆÖs within an organisation can be centralised, hub and spoke with a central PMO with satellite PMOs globally, or embedded within projects. The appropriate structure will be determined by the specific business needs of the organisation. The PMO sits above PM delivery and the supply chain delivery teams.
For further information about the event please click here.N.C. DPI's 2023 Language Diversity Briefing



N.C. DPI's 2023 Language Diversity BriefingMebane Rash
╠²
The number of languages spoken in NC public schools.A PPT Presentation on The Princess and the God: A tale of ancient India by A...



A PPT Presentation on The Princess and the God: A tale of ancient India by A...Beena E S
╠²
A PPT Presentation on The Princess and the God: A tale of ancient India by Aaron ShepardHow to Setup WhatsApp in Odoo 17 - Odoo ║▌║▌▀Żs



How to Setup WhatsApp in Odoo 17 - Odoo ║▌║▌▀ŻsCeline George
╠²
Integrate WhatsApp into Odoo using the WhatsApp Business API or third-party modules to enhance communication. This integration enables automated messaging and customer interaction management within Odoo 17.How to attach file using upload button Odoo 18



How to attach file using upload button Odoo 18Celine George
╠²
In this slide, weŌĆÖll discuss on how to attach file using upload button Odoo 18. Odoo features a dedicated model, 'ir.attachments,' designed for storing attachments submitted by end users. We can see the process of utilizing the 'ir.attachments' model to enable file uploads through web forms in this slide.Lesson Plan M1 2024 Lesson Plan M1 2024 Lesson Plan M1 2024 Lesson Plan M1...



Lesson Plan M1 2024 Lesson Plan M1 2024 Lesson Plan M1 2024 Lesson Plan M1...pinkdvil200
╠²
Lesson Plan M1 2024 Lesson Plan M1 2024 Lesson Plan M1 2024 Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
╠²
Kaun TALHA quiz Prelims - El Dorado 2025



Kaun TALHA quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
╠²
Biosafety-Guidelines & Levels basics.pptx
- 1. BIOSAFETY &BIOETHICS Guidelines & Levels By Dr. S.HIMALINI MARY MATHA COLLEGE OF ARTS & SCIENCES DEPARTMENT OF BIOTECHNOLOGY
- 2. ’éŚ There are several local, state, and federal agencies that either regulate or provide guidelines covering the use of biological agents. ’éŚ Centers for Disease Controls and Prevention (CDC) NationalInstitut es of Health (NIH): Biosafety in Microbiological and Biomedical Laboratories (BMBL)
- 3. ’éŚ 1989 of Environment (Protection) Act 1986, laboratory biosafety through appropriate containment has been identified as the fundamental part of any biological research. ’éŚ In this direction, DBT had earlier published three guidelines namely ŌĆ£Recombinant DNA safety guidelines, 1990ŌĆØ ŌĆ£Revised Guidelines for Safety in Biotechnology, 1994ŌĆØ and ŌĆ£Revised guidelines for research in transgenic plants, 1998ŌĆØ.
- 4. ’éŚ Duringthelasttwodecades,rapidadvancementinbiologyandbiotec hnologyresearch globally and in India, both in public and private sector institutions, necessitated that the above guidelines are reviewed, updated and harmonised with global best practices and guidelines. Further, research on emerging and re-emerging infections and potential risk associated in handling the pathogenic organisms required to put in place stringent yet practical regulations and guidelines for ensuring biosafety measures for protection of public health and environment.
- 5. ’éŚ guidelines for microbiological safe work practices, safety equipment, and facilities that constitute the four established biosafety levels.
- 6. ’éŚ The BMBL is generally considered the standard for biosafety and is the basis for this manual. Compliance with the BMBL is a regulatory requirement for work involving select agents and toxins.
- 7. National Institutes of Health (NIH) ’ü▒Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines). ’ü▒This document provides guidelines for constructing and handling recombinant and synthetic nucleic acid molecules, and organisms containing such nucleic acid. ’ü▒Although these guidelines are not subject to regulatory enforcement (with the exception of work involving select agents and toxins), institutions that receive any NIH funding for research involving recombinant or synthetic nucleic acid molecules are required to comply with these guidelines as a condition of funding.
- 8. ’ü▒This document requires that each institution establish an Institutional Biosafety Committee with the authority to approve proposed research involving recombinant or synthetic nucleic acid molecules, using the NIH Guidelines as a minimum standard.
- 9. Occupational Safety and Health Administration (OSHA) ’ü▒ Blood borne Pathogens. ’ü▒This regulation covers occupational exposure to human blood and other potentially infectious material, including human tissue and cells. OSHA specifies a combination of engineering controls, work practices, and training to reduce the risk of infection. Personnel potentially exposed to human blood and other potentially infectious material must be offered immunization against the Hepatitis B virus and receive annual training
- 10. ’ü▒Personnel who work with HIV or Hepatitis B virus in a research laboratory must receive additional training and demonstrate proficiency in working with human pathogens
- 11. Department of Health and Human Services (CDC) and Department of Agriculture (APHIS) ’ü▒ Select Agent and Toxin Regulations. ’ü▒These regulations cover the possession, use, and transfer of biological agents and toxins that affect humans, animals, and plants and which have been determined to be potential bioterrorism agents (known as select agents). ’ü▒Entities and personnel who wish to work with select agents must be registered with the CDC or APHIS before acquiring or having access to select agents.
- 12. ’ü▒Individuals who require access to select agents require a FBI background check and submittal of fingerprints, and must be approved by the Select Agent Program. ’ü▒These regulations mandate strict requirements for biosafety, emergency planning, and security of select agents and toxins, and requires that laboratories that possess select agents comply with the BMBL (see above) and the OSHA Laboratory Standard
- 13. ’éŚ if select agent toxins are used. Each transfer of a Select Agent must have prior approval of the Select Agent Program through completion of APHIS/CDC Form 2, which requires signature by the Select Agent Responsible Official (University Biosafety Officer) or designated alternate. Accurate inventory records of Select Agents, including transfers, must be maintained.
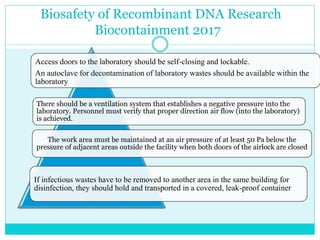
- 14. Biosafety of Recombinant DNA Research Biocontainment 2017 Access doors to the laboratory should be self-closing and lockable. An autoclave for decontamination of laboratory wastes should be available within the laboratory The work area must be maintained at an air pressure of at least 50 Pa below the pressure of adjacent areas outside the facility when both doors of the airlock are closed There should be a ventilation system that establishes a negative pressure into the laboratory. Personnel must verify that proper direction air flow (into the laboratory) is achieved. If infectious wastes have to be removed to another area in the same building for disinfection, they should hold and transported in a covered, leak-proof container
- 15. The facility must have an emergency stop button for the ventilation system, which is easily accessible in case of an emergency. The exhaust air from the facility must pass through a HEPA filter and must be tested by qualified person. Supply or replacement air to the facility must have HEPA filtered. Refrigerators, freezers, incubators, etc. that contain biohazardous materials for storage must be labelled with a biohazard symbol.
- 16. THANK YOU


