Ch2-Cac loai hieu ung-PTSN
- 1. H├│a Hß╗Źc Hß╗»u CŲĪ TS Phan Thanh SŲĪn Nam Bß╗Ö m├┤n Kß╗╣ Thuß║Łt Hß╗»u CŲĪ Khoa Kß╗╣ Thuß║Łt H├│a Hß╗Źc TrŲ░ß╗Øng ─Éß║Īi Hß╗Źc B├Īch Khoa TP. HCM ─Éiß╗ćn thoß║Īi: 8647256 ext. 5681 Email: ptsnam@hcmut.edu.vn 1
- 2. ChŲ░ŲĪng 2: C├üC LOß║ĀI Hiß╗åU ß╗©NG * Hiß╗ću ß╗®ng sß╗▒ dß╗ŗch chuyß╗ān ─æiß╗ćn tß╗Ł trong ph├ón tß╗Ł ß║Żnh hŲ░ß╗¤ng ─æß║┐n cŲĪ chß║┐ phß║Żn ß╗®ng, khß║Ż n─āng phß║Żn ß╗®ng, t├Łnh acid-baseŌĆ” Chia l├Ām 2 loß║Īi: a. Hiß╗ću ß╗®ng ─æiß╗ćn tß╗Ł: ŌĆó HU cß║Żm ß╗®ng I (inductive effect) ŌĆó HU li├¬n hß╗Żp C (conjugation effect) ŌĆó HU si├¬u li├¬n hß╗Żp H (hyperconjugation effect) b. Hiß╗ću ß╗®ng kh├┤ng gian: ŌĆó HU kh├┤ng gian loß║Īi 1 ŌĆó HU kh├┤ng gian loß║Īi 2 2 ŌĆó HU ortho
- 3. I. Hiß╗ću ß╗®ng cß║Żm ß╗®ng I.1. ─Éß╗ŗnh ngh─®a ŌĆó HU cß║Żm ß╗®ng sß╗▒ dß╗ŗch chuyß╗ān ─æiß╗ćn tß╗Ł cß╗¦a c├Īc li├¬n kß║┐t Žā do c├Īc nguy├¬n tß╗Ł trong ph├ón tß╗Ł c├│ ─æß╗Ö ├óm ─æiß╗ćn kh├Īc nhau ph├ón tß╗Ł ph├ón cß╗▒c ŌĆó V├Ł dß╗ź: H H H H C3 C2 C1 Cl H H H ─Éß╗Ö ├óm ─æiß╗ćn Cl > C sß╗▒ dß╗ŗch chuyß╗ān ─ætß╗Ł C1-Cl, C2-C1, C3-C2 3
- 4. I.2. Ph├ón loß║Īi a. HU cß║Żm ß╗®ng dŲ░ŲĪng (+I) ŌĆó G├óy ra bß╗¤i c├Īc nguy├¬n tß╗Ł hay nh├│m nguy├¬n tß╗Ł c├│ khuynh hŲ░ß╗øng nhŲ░ß╗Øng ─æiß╗ćn tß╗Ł b. HU cß║Żm ß╗®ng ├óm (-I) ŌĆó G├óy ra bß╗¤i c├Īc nguy├¬n tß╗Ł hay nh├│m nguy├¬n tß╗Ł c├│ khuynh hŲ░ß╗øng h├║t ─æiß╗ćn tß╗Ł * Quy Ų░ß╗øc: ŌĆó C-H c├│ I = 0 ŌĆó Chiß╗üu chuyß╗ān dß╗ŗch ─ætß╗Ł : ŌĆó Nh├│m nguy├¬n tß╗Ł c├│ khuynh hŲ░ß╗øng nhŲ░ß╗Øng ─æiß╗ćn tß╗Ł > H cho +I (v├Ā ngŲ░ß╗Żc lß║Īi) 4
- 5. I.3. ─Éß║Ęc ─æiß╗ām cß╗¦a HU cß║Żm ß╗®ng ŌĆó C├Īc nguy├¬n tß╗Ł hay nh├│m nguy├¬n tß╗Ł mang ─æiß╗ćn t├Łch + Cho ŌĆōI ŌĆó C├Īc nguy├¬n tß╗Ł hay nh├│m nguy├¬n tß╗Ł mang ─æiß╗ćn t├Łch - cho +I ŌĆó ─Éiß╗ćn t├Łch c├Āng mß║Īnh I c├Āng mß║Īnh, nh├│m nguy├¬n tß╗Ł mang ─æiß╗ćn t├Łch c├│ I mß║Īnh hŲĪn trung h├▓a -N(+)R3 -O(+)R2 -I -O- -N(-)H +I -O(+)R2 > -OR 5
- 6. ŌĆóTrong c├╣ng 1 chu kß╗│ trong bß║Żng HTTH: -I t─āng tß╗½ tr├Īi qua phß║Żi -I: -NR2 < -OR < -F ŌĆóTrong c├╣ng 1 ph├ón nh├│m ch├Łnh : -I giß║Żm tß╗½ tr├¬n xuß╗æng dŲ░ß╗øi -I: -F > -Cl > -Br > -I -I: -OR > -SR > -SeR ŌĆó C├Īc nh├│m alkyl lu├┤n ─æß║®y ─æiß╗ćn tß╗Ł (+I), t─āng dß║¦n tß╗½ bß║Łc 1 ─æß║┐n C bß║Łc 3 +I : -CH3 < -CH2CH3 < -CH(CH3)2 < -C(CH3)3 6
- 7. C├Īc nh├│m kh├┤ng no ─æß╗üu mang ŌĆōI, t─āng dß║¦n theo ─æß╗Ö kh├┤ng no -I: R2C=CR- < < RC C HU cß║Żm ß╗®ng giß║Żm dß║¦n theo mß║Īch C ß║Żnh hŲ░ß╗¤ng ─æß║┐n t├Łnh chß║źt cß╗¦a ph├ón tß╗Ł V├Ł dß╗ź Ka.105 cß╗¦a c├Īc acid: CH3CH2CH2COOH 1.5 CH3CH2CH(Cl)COOH 139 CH3CH(Cl)CH2COOH 8.9 ClCH2CH2CH2COOH 3.0 7
- 8. II. Hiß╗ću ß╗®ng li├¬n hß╗Żp II.1. ─Éß╗ŗnh ngh─®a Hß╗ć li├¬n hß╗Żp: l├Ā nhß╗»ng ph├ón tß╗Ł c├│ li├¬n kß║┐t ŽĆ & ╬▒ ß╗¤ vß╗ŗ tr├Ł lu├ón phi├¬n nhau V├Ł dß╗ź: CH2=CH-CH=CH2 hay CH2=CH-CH=CH-CH=CH2 8
- 9. HU li├¬n hß╗Żp sß╗▒ dß╗ŗch chuyß╗ān ─ætß╗Ł trong 1 hß╗ć li├¬n hß╗Żp, l├Ām cho hß╗ć li├¬n hß╗Żp ─æ├│ trß╗¤ n├¬n ph├ón cß╗▒c V├Ł dß╗ź: CH2=CH-CH=CH2 mß║Łt ─æß╗Ö ─æiß╗ćn tß╗Ł ph├ón bß╗æ ─æß╗üu tr├¬n c├Īc C Tuy nhi├¬n: CH2=CH-CH=CH-CHO ─Éß╗Ö ├óm ─æiß╗ćn cß╗¦a O > C nh├│m C=O sß║Į h├║t ─æiß╗ćn tß╗Ł ŽĆ cß╗¦a hß╗ć ph├ón tß╗Ł trß╗¤ n├¬n ph├ón cß╗▒c ( LH ŽĆ- ŽĆ) 9
- 10. CH2=CH-CH=CH-N(CH3)2 N c├│ ─æ├┤i ─æiß╗ćn tß╗Ł tß╗▒ do (p) c├│ xu hŲ░ß╗øng nhŲ░ß╗Øng ─æiß╗ćn tß╗Ł cho hß╗ć li├¬n hß╗Żp ph├ón tß╗Ł ph├ón cß╗▒c (LH ŽĆ-p) Cl NH2 Li├¬n hß╗Żp ŽĆ-p (-Cl, -NH2 ─æß╗ōng thß╗Øi c├│ ŌĆōI!) 10
- 11. II.2. Ph├ón loß║Īi II.2.1. HU li├¬n hß╗Żp dŲ░ŲĪng (+C) C├Īc ntß╗Ł hay nh├│m nguy├¬n tß╗Ł c├│ khß║Ż n─āng ─æß║®y ─æiß╗ćn tß╗Ł tß╗½ bß║Żn th├ón n├│ ra hß╗ć li├¬n hß╗Żp +C ŌĆó ─Éß║Ęc ─æiß╗ām cß╗¦a +C: a. C├Īc nguy├¬n tß╗Ł hay nh├│m nguy├¬n tß╗Ł c├│ cß║Ęp ─æiß╗ćn tß╗Ł chŲ░a sß╗Ł dß╗źng hoß║Ęc nhß╗»ng ion mang ─æt├Łch (-) ─æß╗üu mang +C -O- -S- -├¢H -├¢R SH SR H NH2 NR2 N C CH3 -F -Cl -Br -I 11 O
- 12. b. C├Īc ion mang ─æiß╗ćn t├Łch ├óm c├│ +C mß║Īnh hŲĪn c├Īc nguy├¬n tß╗Ł trung h├▓a +C: -O- > -OR -S- > -SR c. Trong c├╣ng 1 chu kß╗│ cß╗¦a bß║Żng HTTH: +C giß║Żm tß╗Ł tr├Īi qua phß║Żi +C: -N(R)2 > -OR > -F d. Trong c├╣ng 1 ph├ón nh├│m ch├Łnh: +C giß║Żm tß╗½ tr├¬n xuß╗æng dŲ░ß╗øi +C: -F > -Cl > -Br > -I +C: -OR > -SR > -SeR 12
- 13. II.2.2. HU liß╗ćn hß╗Żp ├óm (-C) C├Īc nguy├¬n tß╗Ł hay nh├│m nguy├¬n tß╗Ł c├│ khß║Ż n─āng h├║t ─æiß╗ćn tß╗Ł cß╗¦a hß╗ć li├¬n hß╗Żp vß╗ü ph├Ła n├│ -C ŌĆó ─Éß║Ęc ─æiß╗ām cß╗¦a ŌĆōC: a. ─Éa sß╗æ c├Īc nh├│m nguy├¬n tß╗Ł mang ŌĆōC l├Ā nhß╗»ng nh├│m kh├┤ng no -NO2 -CN -CHO -COR -COOH -CONH2 13
- 14. b. Trong c├Īc nh├│m C=Z: -C phß╗ź thuß╗Öc Z Z c├│ ─æß╗Ö ├óm ─æiß╗ćn c├Āng lß╗øn, -C c├Āng mß║Īnh -C: C=O > C=NR > C=CR2 c. ─Éß╗æi vß╗øi c├Īc nh├│m nguy├¬n tß╗Ł tŲ░ŲĪng tß╗▒: ─æiß╗ćn t├Łch c├Āng lß╗øn th├¼ ŌĆōC c├Āng mß║Īnh -C: C=N+R2 > C=NR 14
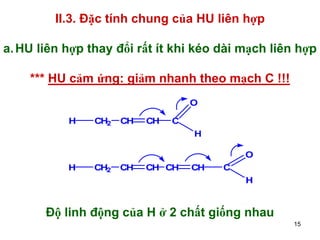
- 15. II.3. ─Éß║Ęc t├Łnh chung cß╗¦a HU li├¬n hß╗Żp a. HU li├¬n hß╗Żp thay ─æß╗Ģi rß║źt ├Łt khi k├®o d├Āi mß║Īch li├¬n hß╗Żp *** HU cß║Żm ß╗®ng: giß║Żm nhanh theo mß║Īch C !!! O H CH2 CH CH C H O H CH2 CH CH CH CH C H ─Éß╗Ö linh ─æß╗Öng cß╗¦a H ß╗¤ 2 chß║źt giß╗æng nhau 15
- 16. Tß╗æc ─æß╗Ö phß║Żn ß╗®ng giß╗æng nhau: OH O O - OH RCHO + H CH2 CH CH C R C CH2 CH CH C H H H O RCHO + H CH2 CH CH CH CH C OH- H OH O R C CH2 CH CH CH CH C H H 16
- 17. b. Mß╗Öt sß╗æ nh├│m thß║┐ chŲ░a no, dß║źu cß╗¦a HU li├¬n hß╗Żp sß║Į thay ─æß╗Ģi t├╣y thuß╗Öc v├Āo nh├│m ntß╗Ł li├¬n kß║┐t vß╗øi n├│ - O O + NH2 N -C6H5: +C -C6H5: -C c. HULH chß╗ē c├│ hiß╗ću lß╗▒c tr├¬n hß╗ć li├¬n hß╗Żp phß║│ng H R C6H5NH2 N C6H5NR2 N H R +C cß╗¦a ŌĆōNR2 giß║Żm so vß╗øi ŌĆōNH2 17
- 18. III. Hiß╗ću ß╗®ng si├¬u li├¬n hß╗Żp III.1. HU si├¬u li├¬n hß╗Żp dŲ░ŲĪng (+H) L├Ā sß╗▒ tŲ░ŲĪng t├Īc cß╗¦a c├Īc ─æiß╗ćn tß╗Ł Žā cß╗¦a li├¬n kß║┐t C╬▒-H vß╗øi hß╗ć ─ætß╗Ł ŽĆ (C=C, -C6H5 ŌĆ”), hoß║Ęc trong carbocation (vd: (CH3)3C+) hay gß╗æc tß╗▒ do (vd: (CH3)3C.) 18
- 19. ŌĆóX├®t phß║Żn ß╗®ng: CH3-CH=CH-CH2-CH3 + HCl Nß║┐u x├®t theo +I: sß║Żn phß║®m ch├Łnh l├Ā: CH3-CH2-CHCl-CH2-CH3 Tuy nhi├¬n, thß╗▒c tß║┐, do t├Īc dß╗źng cß╗¦a +H, sß║Żn phß║®m ch├Łnh l├Ā: H HCl H C CH CH CH2 CH3 CH3 CHCl CH2 CH2 CH3 +╬┤ ŌłÆ╬┤ H 19
- 20. +H c├Āng mß║Īnh khi sß╗æ nguy├¬n tß╗Ł H ß╗¤ C╬▒ c├Āng nhiß╗üu: H H H +H: H C > H3C C H 20
- 21. III.2. HU si├¬u li├¬n hß╗Żp ├óm (-H) L├Ā sß╗▒ tŲ░ŲĪng t├Īc cß╗¦a c├Īc ─ætß╗Ł Žā cß╗¦a lkß║┐t C╬▒-F vß╗øi hß╗ć ─ætß╗Ł ŽĆ (C=C, -C6H5ŌĆ”) F C F F 21
- 22. IV. Hiß╗ću ß╗®ng kh├┤ng gian L├Ā nhß╗»ng loß║Īi hiß╗ću ß╗®ng do k├Łch thŲ░ß╗øc cß╗¦a c├Īc nh├│m thß║┐ trong ph├ón tß╗Ł g├óy n├¬n IV. 1. HU kh├┤ng gian loß║Īi 1 (S1) Do c├Īc nh├│m thß║┐ c├│ k├Łch thŲ░ß╗øc lß╗øn, chiß║┐m 1 khoß║Żng kh├┤ng gian ─æ├Īng kß╗ā cß║Żn trß╗¤ kh├┤ng cho 1 nh├│m chß╗®c n├Āo ─æ├│ trong ph├ón tß╗Ł t├Īc dß╗źng vß╗øi ph├ón tß╗Ł hay ion kh├Īc CH3 CH3 O O + H2N OH HO N O + H2O CH3 CH3 22
- 23. IV. 2. HU kh├┤ng gian loß║Īi 2 (S2) Do c├Īc nh├│m thß║┐ c├│ k├Łch thŲ░ß╗øc lß╗øn hß╗ć li├¬n hß╗Żp bß╗ŗ mß║źt t├Łnh phß║│ng kh├┤ng cho 1 sß╗æ phß║Żn ß╗®ng Xß║Ży ra R R H3C H3C N + Cl-N N+ N N N H3C H3C R R ŌĆó R = H: phß║Żn ß╗®ng xß║Ży ra ŌĆó R=-CH3: hß╗ć li├¬n hß╗Żp mß║źt t├Łnh phß║│ng +C cß╗¦a ŌĆōN(CH3)2 giß║Żm mß║Īnh phß║Żn ß╗®ng kh├┤ng xß║Ży ra 23
- 24. IV. 3. Hiß╗ću ß╗®ng Ortho G├óy ra bß╗¤i c├Īc nh├│m thß║┐ ß╗¤ vß╗ŗ tr├Ł ortho trong v├▓ng benzene g├óy ß║Żnh hŲ░ß╗¤ng ─æß║Ęc biß╗ćt so vß╗øi c├Īc nh├│m thß║┐ ß╗¤ vß╗ŗ tr├Ł kh├Īc HU ortho: hß╗Śn hß╗Żp cß╗¦a nhiß╗üu yß║┐u tß╗æ (S1, S2, I, li├¬n kß║┐t H) 24
- 25. X├®t hß║▒ng sß╗æ ph├ón ly (Ka.105) cß╗¦a dß║½n xuß║źt cß╗¦a benzoic acid C6H4(R)COOH Vß╗ŗ tr├Ł / R OH F NO2 o- 10.5 54.4 67.1 m- 8.3 13.7 32.1 p- 2.9 7.2 37.6 LŲ░u ├Į: -I cß╗¦a NO2 > -I cß╗¦a F 25
- 26. T├Łnh acid: H H H O O O O O O C H C C O > > OH OH ŌĆóo-: OH c├│ ŌĆōI h├║t ─ætß╗Ł & li├¬n kß║┐t H O-H trong COOH ph├ón cß╗▒c mß║Īnh nhß║źt ŌĆóp-, m-: OH c├│ ŌĆōI h├║t ─æiß╗ćn tß╗Ł nhŲ░ng -I giß║Żm dß║¦n theo chiß╗üu d├Āi mß║Īch C O-H trong COOH ß╗¤ p- ├Łt bß╗ŗ ph├ón cß╗▒c nhß║źt ŌĆólŲ░u ├Į: OH trong o- & p- c├│ +C ─æß║®y ─æiß╗ćn tß╗Ł l├¬n hß╗ć li├¬n hß╗Żp p-Žā-ŽĆ- Žā ŌĆ”C=O trong m-: hß╗ć li├¬n hß╗Żp n├Āy bß╗ŗ ─æß╗®t ─æoß║Īn do Žā- Žā li├¬n tß╗źc !!! c├Āng 26 l├Ām cho t├Łnh acid cß╗¦a m- > p-
- 27. ŌĆóT├Łnh acid cß╗¦a C6H4(F)COOH: o- > m- > p- do ŌĆōI giß║Żm theo chiß╗üu d├Āi mß║Īch C Khß║Ż n─āng h├║t (-I) hay ─æß║®y (+C) ─æiß╗ćn tß╗Ł cß╗¦a ŌĆōF, Cl, Br, I: -I > +C ŌĆóT├Łnh acid cß╗¦a C6H4(NO2)COOH: o- > p- > m- 27
- 28. - - O +O O +O N N H O OH o-nitrophenol: li├¬n kß║┐t H nß╗Öi ph├ón tß╗Ł tos├┤i thß║źp, kh├┤ng tan trong nŲ░ß╗øc c├│ thß╗ā chŲ░ng l├┤i cuß╗æn hŲĪi nŲ░ß╗øc p-nitrophenol: chß╗ē c├│ li├¬n kß║┐t H ngoß║Īi ph├ón tß╗Ł trong nŲ░ß╗øc tan tß╗æt trong nŲ░ß╗øc, tos├┤i cao 28
- 29. V. ß║ónh hŲ░ß╗¤ng cß╗¦a c├Īc hiß╗ću ß╗®ng l├¬n t├Łnh acid ŌĆō base v├Ā ─æß╗Ö bß╗ün cß╗¦a carbocation V.1. ß║ónh hŲ░ß╗¤ng cß╗¦a HU cß║Żm ß╗®ng l├¬n t├Łnh acid ŌĆó C├Īc R-OH, R-COOH c├│ chß╗®a nh├│m thß║┐ c├│ +I t├Łnh acid giß║Żm ŌĆó Chß╗®a nh├│m thß║┐ c├│ ŌĆōI: t├Łnh acid t─āng do O-H c├Āng ph├ón cß╗▒c 29
- 30. T├Łnh acid cß╗¦a c├Īc acid: F3C-COOH (pKa 0.23) > Cl3C-COOH (0.66) > Cl2CH-COOH (1.25) > NO2-CH2-COOH (1.68) > NC-CH2-COOH (2.47) > F-CH2-COOH (2.57) > Cl-CH2-COOH (2.87) > Br-CH2-COOH (2.90) > HCOOH (3.75) > HO-CH2-COOH (3.83) > CH3COOH (4.76) > CH3CH2COOH (4.87) > (CH3)3C-COOH (5.03) 30
- 31. Nh├│m thß║┐ c├Āng xa C╬▒ ß║Żnh hŲ░ß╗¤ng c├Āng yß║┐u do I giß║Żm mß║Īnh: T├Łnh acid: F3C-COOH > F3C-CH2-COOH > F3C-CH2-CH2-COOH 31
- 32. V.2. ß║ónh hŲ░ß╗¤ng cß╗¦a HU li├¬n hß╗Żp, HU si├¬u li├¬n hß╗Żp l├¬n t├Łnh acid ŌĆó T├Łnh acid cß╗¦a alcohol < phenol ŌĆó Nh├│m thß║┐ c├│ ŌĆōC sß║Į l├Ām t─āng t├Łnh acid & ngŲ░ß╗Żc lß║Īi - T├Łnh acid: +C, -I O +O H N -I, -C +I H H C H +H, +I H NH2 C > > H > > O O O O O H H H H H Th├┤ng thŲ░ß╗Øng (kh├┤ng lu├┤n lu├┤n!) : C > H > I 32
- 33. a. Acid b├®o kh├┤ng no: ŌĆó T├Łnh acid mß║Īnh hŲĪn acid no c├╣ng mß║Īch C (do C=C c├│ ŌĆōI) ŌĆó Nß╗æi ─æ├┤i C=C c├Āng gß║¦n ŌĆōCOOH th├¼ t├Łnh acid c├Āng mß║Īnh ŌĆó Tuy nhi├¬n: nß║┐u C=C li├¬n hß╗Żp vß╗øi C=O trong ŌĆō COOH th├¼ t├Łnh acid giß║Żm do +C cß╗¦a C=C!!! ŌĆó T├Łnh acid: CH3-CH=CH-CH2-COOH (pKa 4.48) > CH2=CH-CH2-CH2-COOH (4.68) > CH3-CH2-CH=CH-COOH (4.83) 33
- 34. ŌĆó Nß╗æi ba CŌēĪC cho d├╣ ß╗¤ vß╗ŗ tr├Ł li├¬n hß╗Żp vß╗øi C=O th├¼ vß║½n l├Ām t─āng mß║Īnh t├Łnh acid (kh├Īc C=C): do ŌĆōI cß╗¦a CŌēĪC mß║Īnh & chß╗ē c├│ 1 lkß║┐t ŽĆ cß╗¦a CŌēĪC cho +C li├¬n hß╗Żp vß╗øi C=O, lkß║┐t ŽĆ c├▓n lß║Īi cho ŌĆōI nhŲ░ng kh├┤ng c├│ +C!!! ŌĆó T├Łnh acid: CHŌēĪC-COOH (pKa 1.84) > CH3-CŌēĪC-COOH (2.60) > CH2=CH-COOH (4.25) 34
- 35. b. Acid c├│ v├▓ng thŲĪm: ŌĆóT├Łnh acid H-COOH (pKa 3.75) > C6H5-COOH (4.18) do +C cß╗¦a C6H5- mß║Īnh hŲĪn ŌĆōI ŌĆóT├Łnh acid t├╣y thuß╗Öc bß║Żn chß║źt & vß╗ŗ tr├Ł nh├│m thß║┐: o-NO2-C6H5-COOH > p- > m- ŌĆó Halogen cho ŌĆōI > +C o-Cl-C6H5-COOH > m- > p- 35
- 36. V.3. ß║ónh hŲ░ß╗¤ng l├¬n t├Łnh base ŌĆó Mß║Łt ─æß╗Ö ─æiß╗ćn tß╗Ł tr├¬n N c├Āng lß╗øn t├Łnh base cß╗¦a amine c├Āng mß║Īnh ŌĆó Nh├│m thß║┐ ─æß║®y ─æiß╗ćn tß╗Ł (+I) sß║Į l├Ām t─āng t├Łnh base cß╗¦a amine & ngŲ░ß╗Żc lß║Īi (-I, -C) T├Łnh base: (CH3)2NH > CH3NH2 > NH3 > C6H5NH2 > p-NO2-C6H4-NH2 36
- 37. ŌĆó T├Łnh base: p-NO2-C6H4-NH2 < m- NO2-C6H4-NH2 < p-Cl-C6H4-NH2 < C6H5-NH2 < p-CH3O-C6H4-NH2 p-NO2: -I, -C mß║Īnh nhß║źt, m-NO2: -I mß║Īnh, -C kh├┤ng ß║Żnh hŲ░ß╗¤ng nhiß╗üu do hß╗ć li├¬n hß╗Żp bß╗ŗ ─æß╗®t ─æoß║Īn -Cl: -I mß║Īnh hŲĪn +C, -I yß║┐u hŲĪn -NO2 p-CH3O: +C mß║Īnh hŲĪn ŌĆōI mß║Łt ─æß╗Ö ─æiß╗ćn tß╗Ł tr├¬n N cao nhß║źt base mß║Īnh nhß║źt ŌĆó Acid li├¬n hß╗Żp c├Āng yß║┐u th├¼ t├Łnh base c├Āng mß║Īnh T├Łnh base: HCŌēĪC- > (CH3)3CO- > CH3O- > OH- > C6H5O- > CH3COO- 37
- 38. V.4. ß║ónh hŲ░ß╗¤ng l├¬n ─æß╗Ö bß╗ün cß╗¦a c├Īc carbocation ŌĆó ─Éiß╗ćn t├Łch dŲ░ŲĪng tr├¬n c├Īc cation c├Āng ─æŲ░ß╗Żc giß║Żi tß╗Åa (c├Āng nhß╗Å) th├¼ cation c├Āng bß╗ün ŌĆó ─Éß╗Ö bß╗ün do hiß╗ću ß╗®ng ─æß║®y ─æiß╗ćn tß╗Ł cß╗¦a +H, +I: H H H H + < HH C H H C CH2 < H C C H C C+ H HH C H HH C H H H 38
- 39. ─Éß╗Ö bß╗ün cß╗¦a carbocation: (CH3)3C+ < C6H5CH2+ < (C6H5)2CH+ Do +C cß╗¦a -C6H5 mß║Īnh hŲĪn +I, +H cß╗¦a ŌĆōCH3 ─Éiß╗ćn t├Łch c├Āng ─æŲ░ß╗Żc giß║Żi tß╗Åa carbocation c├Āng bß╗ün 39
- 40. ŌĆó ─Éß╗Ö bß╗ün cß╗¦a carbocation: H H C CH2 < H3C O CH2 < H3C NH CH2 H +C cß╗¦a ŌĆōNH- > +C cß╗¦a ŌĆōO- > +H & I cß╗¦a ŌĆōCH3 -NH- & -O- ─æß╗ōng thß╗Øi c├│ ŌĆōI nhŲ░ng +C ß║Żnh hŲ░ß╗¤ng mß║Īnh hŲĪn -NH- giß║Żi tß╗Åa ─æt├Łch dŲ░ŲĪng mß║Īnh nhß║źt bß╗ün nhß║źt ŌĆó Gß╗æc allyl CH2=CH-CH2+ hay C6H5-CH2+ rß║źt bß╗ün do +C cß╗¦a vinyl hay phenyl 40















































![Bt hoa huu_co_tap1[1]](https://cdn.slidesharecdn.com/ss_thumbnails/bthoahuucotap11-160622074123-thumbnail.jpg?width=560&fit=bounds)

























![[123doc.vn] hhc daicuong](https://cdn.slidesharecdn.com/ss_thumbnails/123doc-140926112909-phpapp01-thumbnail.jpg?width=560&fit=bounds)
























![[Hoa hocthpt]onthidaihocphandienli](https://cdn.slidesharecdn.com/ss_thumbnails/hoahocthptonthidaihocphandienli-140620003006-phpapp02-thumbnail.jpg?width=560&fit=bounds)







