ionic equation and net ionic equation PPT
Download as PPT, PDF0 likes15 views
ionic equation and net ionic equation
1 of 8
Download to read offline



Recommended
Ch4 Reactions in Aqueous Solution



Ch4 Reactions in Aqueous SolutionSa'ib J. Khouri
╠²
This document discusses various topics related to aqueous solutions and reactions. It begins by defining key terms like solute, solvent, electrolyte and providing examples. It then covers properties of aqueous solutions such as conductivity. Various acid-base reactions and concepts are explained like Br├Ėnsted-Lowry acids and bases, neutralization reactions. Oxidation-reduction reactions and oxidation numbers are also discussed. Finally, the document covers concentration of solutions and calculations involving molarity, dilution and preparation of solutions.Chapter 4 Lecture- Solution Stoich



Chapter 4 Lecture- Solution StoichMary Beth Smith
╠²
This document provides information on various chemistry concepts related to solutions and reactions in aqueous solutions. It defines key terms like electrolytes, nonelectrolytes, dissociation, and precipitation reactions. It also discusses acid-base reactions and neutralization reactions. Oxidation-reduction reactions and displacement reactions are introduced. Molarity is defined as a way to quantify concentration in solutions.Net ionic equations



Net ionic equationszehnerm2
╠²
The document discusses net ionic equations, which involve writing molecular and ionic equations and identifying spectator ions. A molecular equation shows all species as whole units, while an ionic equation shows dissolved species as free ions. To write a net ionic equation, the molecular equation is first written and balanced, then molecules are broken into ions. Spectator ions that are present on both sides of the reaction are then canceled to give the net ionic equation. The document also discusses what substances will ionize or dissociate into ions in solution based on their type (salt, acid, base) and whether they are considered strong electrolytes.Lab 4



Lab 4dluetgens
╠²
This document discusses different types of chemical reactions including decomposition, synthesis, combustion, double replacement, and single replacement reactions. It provides examples of each type of reaction and explains the key features that define them. Double replacement reactions are highlighted, where a metal replaces a metal in a compound and a nonmetal replaces a nonmetal, forming a precipitate if one of the products is insoluble. Guidelines are provided for writing molecular, total ionic, and net ionic equations for double replacement reactions.Chapter 8: Reactions in Aqueous Solution



Chapter 8: Reactions in Aqueous SolutionMelissa McDonald
╠²
This chapter discusses different types of chemical reactions in aqueous solutions. It introduces driving forces that cause reactions, such as formation of a solid, water, or gas. It explains how to predict products using solubility rules and oxidation-reduction reactions when metals react with nonmetals. Reactions are classified into double displacement, acid-base, single replacement, combustion, synthesis, or decomposition reactions based on their driving forces.Science 10th Class 



Science 10th Class Rahul Thakur
╠²
This document provides information on stoichiometry, which involves using mole ratios from balanced chemical equations to calculate mass relationships between substances in a chemical reaction. It outlines the steps to solve stoichiometry problems, which include writing a balanced equation, identifying known and unknown quantities, setting up mole ratio conversion factors between moles of reactants and products, and checking the answer. Key concepts discussed include the mole ratio from coefficients in a balanced equation, molar mass to convert between moles and grams, and the molar volume used to calculate liters of gas at standard temperature and pressure.Ch21 electrochem 6e_final



Ch21 electrochem 6e_finalPeterEdward21
╠²
This document provides an overview of electrochemistry and voltaic cells. It discusses redox reactions, how to balance redox reactions using the half-reaction method, and the components and operation of voltaic cells. Specifically, it explains that a voltaic cell uses a spontaneous redox reaction to generate electrical energy by separating the oxidation and reduction half-reactions into two half-cells connected by an external circuit and salt bridge. Electrons flow from the anode, where oxidation occurs, through the external circuit to the cathode, where reduction occurs.REDOX REACTION



REDOX REACTIONArvind Singh Heer
╠²
This document provides an overview of redox reactions including:
- Redox reactions involve the transfer of electrons between chemical species, resulting in oxidation and reduction.
- Oxidizing agents gain electrons and are reduced, while reducing agents lose electrons and are oxidized.
- Latimer, Frost, and Pourbaix diagrams can be used to predict and understand redox reactions in aqueous solutions by showing the thermodynamic stability of different oxidation states.
- Key concepts like disproportionation, oxidizing/reducing abilities, and stable/unstable species can be determined from these types of diagrams.B.tech. ii engineering chemistry unit 5 A electrochemistry



B.tech. ii engineering chemistry unit 5 A electrochemistryRai University
╠²
Arrhenius proposed the theory of electrolytic dissociation to explain the properties of electrolytic solutions. The theory states that when an electrolyte dissolves in water, it breaks up into ions - positively charged cations and negatively charged anions. This process is called ionization. Ions are constantly recombining and dissociating, reaching a state of dynamic equilibrium. The extent of ionization depends on an equilibrium constant. Strong electrolytes have a high equilibrium constant and ionize completely, while weak electrolytes have a low constant and only partially ionize.New chm 152 unit 7 power points su13



New chm 152 unit 7 power points su13caneman1
╠²
The document discusses oxidation-reduction (redox) reactions and provides information on key concepts:
- Oxidation involves loss of electrons and an increase in oxidation number, while reduction involves gain of electrons and a decrease in oxidation number.
- Redox reactions involve both oxidation and reduction halves that occur simultaneously.
- The half-reaction method is used to balance redox reactions, by separating the reaction into oxidation and reduction halves and balancing atoms, charges, and electrons between the halves.New chm 152 unit 7 power points su13



New chm 152 unit 7 power points su13caneman1
╠²
The document discusses oxidation-reduction (redox) reactions and provides information on key concepts:
- Oxidation involves loss of electrons and increases oxidation number, reduction involves gain of electrons and decreases oxidation number.
- Redox reactions involve both oxidation and reduction occurring simultaneously.
- Oxidizing agents are reduced by gaining electrons from other substances, while reducing agents are oxidized by losing electrons to other substances.Lect w13 152_electrochemistry_abbrev



Lect w13 152_electrochemistry_abbrevchelss
╠²
The document discusses an electrochemistry unit covering chemical reactions that produce electrical currents or voltages. It provides information on voltaic cells, also known as galvanic cells, which harness spontaneous redox reactions to generate electricity. The document explains that voltaic cells use two half-reactions, an oxidation reaction at the anode and a reduction reaction at the cathode, to drive electrons from the anode to the cathode through an external circuit. Standard reduction potentials are used to predict if reactions will occur spontaneously.AP Chemistry Chapter 4 Outline



AP Chemistry Chapter 4 OutlineJane Hamze
╠²
This document summarizes key concepts in solution chemistry and stoichiometry, including:
1) Solutions, electrolytes, dissociation, and precipitation reactions are discussed. Strong and weak electrolytes are defined.
2) Acid-base reactions such as neutralization and gas-forming reactions are covered. Oxidation-reduction reactions and oxidation numbers are also introduced.
3) Concepts like molarity, dilution, and titration are explained as methods to quantify concentrations in solutions and chemical reactions.Ch04 outline



Ch04 outlineAP_Chem
╠²
This document summarizes key concepts in solution chemistry and stoichiometry, including:
1) Solutions, electrolytes, dissociation, and precipitation reactions are discussed. Strong and weak electrolytes are defined.
2) Acid-base reactions such as neutralization and gas-forming reactions are covered. Oxidation-reduction reactions and displacement reactions are also summarized.
3) Concepts including molarity, dilution, and titration reactions are introduced for quantitative chemical calculations.Tutorial 2- Balancing Chemical Equations.ppt



Tutorial 2- Balancing Chemical Equations.pptjameiljrmagomnang1
╠²
Okay, here are the steps to balance this reaction:
Step 1) Identify oxidizing and reducing agents:
MnO4- is reduced, so it is the oxidizing agent.
MnO4- + 5e- ŌåÆ Mn2+
SO2 is oxidized, so it is the reducing agent.
SO2 ŌåÆ SO42- + 4e-
Step 2) Balance other elements: No need here.
Step 3) Balance O by adding H2O:
MnO4- + 5e- ŌåÆ Mn2+ + 4H2O
SO2 ŌåÆ SO42- + 4e-
Step 4) Balance H by adding H+:
MCh4 Reactions in Aqueous Solution (updated)



Ch4 Reactions in Aqueous Solution (updated)Sa'ib J. Khouri
╠²
A document discusses various topics relating to chemistry solutions including:
1) The definition of solutions, solvents, and solutes. A solution is a homogeneous mixture of substances where the solute is the smaller component dissolved in the solvent.
2) Properties of aqueous solutions including that electrolytes can conduct electricity while nonelectrolytes cannot. Strong electrolytes dissociate completely while weak electrolytes only partially dissociate.
3) Reactions involving solutions such as precipitation reactions, acid-base reactions, and redox reactions. Precipitation occurs when an insoluble solid forms. Acid-base reactions involve acids and bases reacting to form water and a salt. Redox reactions involve the transfer of electrons8 electrochemistry



8 electrochemistryUniversity of Zambia, School of Pharmacy, Lusaka, Zambia
╠²
The document provides an introduction to key concepts in electrochemistry including oxidation/reduction reactions, oxidation numbers, and definitions of terms like oxidizing agent and reducing agent. It then discusses rules for assigning oxidation numbers, types of redox reactions like disproportionation, electrochemical cells, and how to determine the potential of a cell.Redox



RedoxFer Pelaez
╠²
This document discusses oxidation-reduction (redox) reactions through examples of writing complete and net ionic equations, identifying oxidizing and reducing agents, writing half-reactions, and balancing redox reactions. Key points covered include:
1. Redox reactions involve the transfer of electrons between atoms.
2. Net ionic equations show the ionic form of the reactants and products.
3. The atom that loses electrons is oxidized and acts as the reducing agent. The atom that gains electrons is reduced and acts as the oxidizing agent.
4. Half-reactions allow identifying how many electrons are lost or gained in the oxidation and reduction steps.
5. Balancing redox reactionsChapter 8 Notes



Chapter 8 NotesNorth PIke High School
╠²
The document discusses chemical reactions and equations. It provides information on:
- Writing balanced chemical equations to represent reactions
- Indications that a reaction occurred like heat/gas production
- Characteristics of chemical equations like conservation of mass
- Examples of balancing equations and writing equations for reactionsChapter 4 - Precipitation Reactions.pptx



Chapter 4 - Precipitation Reactions.pptxmichael547874
╠²
This document discusses precipitation reactions and provides information on:
- Types of precipitation reactions and how to determine if a product is soluble or insoluble using solubility rules.
- How to predict if a precipitation reaction will occur by assigning oxidation states, writing molecular, complete ionic, and net ionic equations.
- How to perform stoichiometric calculations involving precipitation reactions, including determining moles or mass of reactants and products.
- Key concepts related to solutions including molarity, using molarity in calculations, dilution, and limiting reagents.AP Chemistry Chapter 4 Sample Exercise



AP Chemistry Chapter 4 Sample ExerciseJane Hamze
╠²
The document provides sample exercises to practice writing chemical equations and determining oxidation states. It includes questions about relating numbers of ions to chemical formulas, using solubility rules to classify compounds, predicting precipitation reactions, writing molecular and net ionic equations, identifying strong/weak electrolytes, and determining oxidation numbers of sulfur in various compounds. The exercises are accompanied by explanations of the thought processes and steps to arrive at the answers.Acids and bases dr.surendran prambadath



Acids and bases dr.surendran prambadathSurendran Parambadath
╠²
The document discusses acids and bases according to different theories including Arrhenius, Bronsted-Lowry, and Lewis concepts. It defines acids and bases, describes their properties, and explains neutralization reactions. Examples are provided of strong vs weak acids and bases as well as monoprotic, diprotic, and triprotic acids and bases based on their equivalent weights.6.1-a)---b)-Redox-Processes--Half-Equations-and-Oxidation-States.pptx



6.1-a)---b)-Redox-Processes--Half-Equations-and-Oxidation-States.pptxJinsyAjish2
╠²
This document discusses redox reactions in terms of electron transfer and oxidation numbers. It defines redox reactions as reactions where one species is reduced while another is oxidized simultaneously through electron transfer. It explains how to write half reactions showing oxidation and reduction and how to balance them to obtain an overall ionic or full reaction equation. It also provides rules for determining the oxidation state or number of atoms in compounds and examples of oxidation states.Titration-curves.pptx



Titration-curves.pptxJinsyAjish2
╠²
This document provides information about acid-base titrations including definitions of key terms like equivalence point and neutral point. It describes what happens during a titration and the typical shape of the titration curve. It explains that the titration curve for a strong acid with a strong base will have a sharp pH rise at the equivalence point, making phenolphthalein or methyl orange suitable indicators. The curve for a weak acid and weak base does not have a well-defined equivalence point, so neither indicator is suitable.More Related Content
Similar to ionic equation and net ionic equation PPT (17)
Ch21 electrochem 6e_final



Ch21 electrochem 6e_finalPeterEdward21
╠²
This document provides an overview of electrochemistry and voltaic cells. It discusses redox reactions, how to balance redox reactions using the half-reaction method, and the components and operation of voltaic cells. Specifically, it explains that a voltaic cell uses a spontaneous redox reaction to generate electrical energy by separating the oxidation and reduction half-reactions into two half-cells connected by an external circuit and salt bridge. Electrons flow from the anode, where oxidation occurs, through the external circuit to the cathode, where reduction occurs.REDOX REACTION



REDOX REACTIONArvind Singh Heer
╠²
This document provides an overview of redox reactions including:
- Redox reactions involve the transfer of electrons between chemical species, resulting in oxidation and reduction.
- Oxidizing agents gain electrons and are reduced, while reducing agents lose electrons and are oxidized.
- Latimer, Frost, and Pourbaix diagrams can be used to predict and understand redox reactions in aqueous solutions by showing the thermodynamic stability of different oxidation states.
- Key concepts like disproportionation, oxidizing/reducing abilities, and stable/unstable species can be determined from these types of diagrams.B.tech. ii engineering chemistry unit 5 A electrochemistry



B.tech. ii engineering chemistry unit 5 A electrochemistryRai University
╠²
Arrhenius proposed the theory of electrolytic dissociation to explain the properties of electrolytic solutions. The theory states that when an electrolyte dissolves in water, it breaks up into ions - positively charged cations and negatively charged anions. This process is called ionization. Ions are constantly recombining and dissociating, reaching a state of dynamic equilibrium. The extent of ionization depends on an equilibrium constant. Strong electrolytes have a high equilibrium constant and ionize completely, while weak electrolytes have a low constant and only partially ionize.New chm 152 unit 7 power points su13



New chm 152 unit 7 power points su13caneman1
╠²
The document discusses oxidation-reduction (redox) reactions and provides information on key concepts:
- Oxidation involves loss of electrons and an increase in oxidation number, while reduction involves gain of electrons and a decrease in oxidation number.
- Redox reactions involve both oxidation and reduction halves that occur simultaneously.
- The half-reaction method is used to balance redox reactions, by separating the reaction into oxidation and reduction halves and balancing atoms, charges, and electrons between the halves.New chm 152 unit 7 power points su13



New chm 152 unit 7 power points su13caneman1
╠²
The document discusses oxidation-reduction (redox) reactions and provides information on key concepts:
- Oxidation involves loss of electrons and increases oxidation number, reduction involves gain of electrons and decreases oxidation number.
- Redox reactions involve both oxidation and reduction occurring simultaneously.
- Oxidizing agents are reduced by gaining electrons from other substances, while reducing agents are oxidized by losing electrons to other substances.Lect w13 152_electrochemistry_abbrev



Lect w13 152_electrochemistry_abbrevchelss
╠²
The document discusses an electrochemistry unit covering chemical reactions that produce electrical currents or voltages. It provides information on voltaic cells, also known as galvanic cells, which harness spontaneous redox reactions to generate electricity. The document explains that voltaic cells use two half-reactions, an oxidation reaction at the anode and a reduction reaction at the cathode, to drive electrons from the anode to the cathode through an external circuit. Standard reduction potentials are used to predict if reactions will occur spontaneously.AP Chemistry Chapter 4 Outline



AP Chemistry Chapter 4 OutlineJane Hamze
╠²
This document summarizes key concepts in solution chemistry and stoichiometry, including:
1) Solutions, electrolytes, dissociation, and precipitation reactions are discussed. Strong and weak electrolytes are defined.
2) Acid-base reactions such as neutralization and gas-forming reactions are covered. Oxidation-reduction reactions and oxidation numbers are also introduced.
3) Concepts like molarity, dilution, and titration are explained as methods to quantify concentrations in solutions and chemical reactions.Ch04 outline



Ch04 outlineAP_Chem
╠²
This document summarizes key concepts in solution chemistry and stoichiometry, including:
1) Solutions, electrolytes, dissociation, and precipitation reactions are discussed. Strong and weak electrolytes are defined.
2) Acid-base reactions such as neutralization and gas-forming reactions are covered. Oxidation-reduction reactions and displacement reactions are also summarized.
3) Concepts including molarity, dilution, and titration reactions are introduced for quantitative chemical calculations.Tutorial 2- Balancing Chemical Equations.ppt



Tutorial 2- Balancing Chemical Equations.pptjameiljrmagomnang1
╠²
Okay, here are the steps to balance this reaction:
Step 1) Identify oxidizing and reducing agents:
MnO4- is reduced, so it is the oxidizing agent.
MnO4- + 5e- ŌåÆ Mn2+
SO2 is oxidized, so it is the reducing agent.
SO2 ŌåÆ SO42- + 4e-
Step 2) Balance other elements: No need here.
Step 3) Balance O by adding H2O:
MnO4- + 5e- ŌåÆ Mn2+ + 4H2O
SO2 ŌåÆ SO42- + 4e-
Step 4) Balance H by adding H+:
MCh4 Reactions in Aqueous Solution (updated)



Ch4 Reactions in Aqueous Solution (updated)Sa'ib J. Khouri
╠²
A document discusses various topics relating to chemistry solutions including:
1) The definition of solutions, solvents, and solutes. A solution is a homogeneous mixture of substances where the solute is the smaller component dissolved in the solvent.
2) Properties of aqueous solutions including that electrolytes can conduct electricity while nonelectrolytes cannot. Strong electrolytes dissociate completely while weak electrolytes only partially dissociate.
3) Reactions involving solutions such as precipitation reactions, acid-base reactions, and redox reactions. Precipitation occurs when an insoluble solid forms. Acid-base reactions involve acids and bases reacting to form water and a salt. Redox reactions involve the transfer of electrons8 electrochemistry



8 electrochemistryUniversity of Zambia, School of Pharmacy, Lusaka, Zambia
╠²
The document provides an introduction to key concepts in electrochemistry including oxidation/reduction reactions, oxidation numbers, and definitions of terms like oxidizing agent and reducing agent. It then discusses rules for assigning oxidation numbers, types of redox reactions like disproportionation, electrochemical cells, and how to determine the potential of a cell.Redox



RedoxFer Pelaez
╠²
This document discusses oxidation-reduction (redox) reactions through examples of writing complete and net ionic equations, identifying oxidizing and reducing agents, writing half-reactions, and balancing redox reactions. Key points covered include:
1. Redox reactions involve the transfer of electrons between atoms.
2. Net ionic equations show the ionic form of the reactants and products.
3. The atom that loses electrons is oxidized and acts as the reducing agent. The atom that gains electrons is reduced and acts as the oxidizing agent.
4. Half-reactions allow identifying how many electrons are lost or gained in the oxidation and reduction steps.
5. Balancing redox reactionsChapter 8 Notes



Chapter 8 NotesNorth PIke High School
╠²
The document discusses chemical reactions and equations. It provides information on:
- Writing balanced chemical equations to represent reactions
- Indications that a reaction occurred like heat/gas production
- Characteristics of chemical equations like conservation of mass
- Examples of balancing equations and writing equations for reactionsChapter 4 - Precipitation Reactions.pptx



Chapter 4 - Precipitation Reactions.pptxmichael547874
╠²
This document discusses precipitation reactions and provides information on:
- Types of precipitation reactions and how to determine if a product is soluble or insoluble using solubility rules.
- How to predict if a precipitation reaction will occur by assigning oxidation states, writing molecular, complete ionic, and net ionic equations.
- How to perform stoichiometric calculations involving precipitation reactions, including determining moles or mass of reactants and products.
- Key concepts related to solutions including molarity, using molarity in calculations, dilution, and limiting reagents.AP Chemistry Chapter 4 Sample Exercise



AP Chemistry Chapter 4 Sample ExerciseJane Hamze
╠²
The document provides sample exercises to practice writing chemical equations and determining oxidation states. It includes questions about relating numbers of ions to chemical formulas, using solubility rules to classify compounds, predicting precipitation reactions, writing molecular and net ionic equations, identifying strong/weak electrolytes, and determining oxidation numbers of sulfur in various compounds. The exercises are accompanied by explanations of the thought processes and steps to arrive at the answers.Acids and bases dr.surendran prambadath



Acids and bases dr.surendran prambadathSurendran Parambadath
╠²
The document discusses acids and bases according to different theories including Arrhenius, Bronsted-Lowry, and Lewis concepts. It defines acids and bases, describes their properties, and explains neutralization reactions. Examples are provided of strong vs weak acids and bases as well as monoprotic, diprotic, and triprotic acids and bases based on their equivalent weights.More from JinsyAjish2 (12)
6.1-a)---b)-Redox-Processes--Half-Equations-and-Oxidation-States.pptx



6.1-a)---b)-Redox-Processes--Half-Equations-and-Oxidation-States.pptxJinsyAjish2
╠²
This document discusses redox reactions in terms of electron transfer and oxidation numbers. It defines redox reactions as reactions where one species is reduced while another is oxidized simultaneously through electron transfer. It explains how to write half reactions showing oxidation and reduction and how to balance them to obtain an overall ionic or full reaction equation. It also provides rules for determining the oxidation state or number of atoms in compounds and examples of oxidation states.Titration-curves.pptx



Titration-curves.pptxJinsyAjish2
╠²
This document provides information about acid-base titrations including definitions of key terms like equivalence point and neutral point. It describes what happens during a titration and the typical shape of the titration curve. It explains that the titration curve for a strong acid with a strong base will have a sharp pH rise at the equivalence point, making phenolphthalein or methyl orange suitable indicators. The curve for a weak acid and weak base does not have a well-defined equivalence point, so neither indicator is suitable.Empirical_Formulae_LO.pptx



Empirical_Formulae_LO.pptxJinsyAjish2
╠²
This document outlines the process for calculating empirical formulas. It begins by defining an empirical formula as the simplest ratio of atoms in a compound. It then provides an example calculation for a compound containing 27.3% carbon and 72.7% oxygen. The steps involve determining the number of moles of each element and dividing by the smallest number of moles to obtain the empirical formula, which in this case is CO2. The document also mentions calculating the empirical formula of copper sulfate and assessing work at different grade levels.Titrations-step-by-step-TES.pptx



Titrations-step-by-step-TES.pptxJinsyAjish2
╠²
This document provides information and examples related to titration calculations. It defines key terms like indicators, acids, alkalis and salts. It also outlines the step-by-step method for carrying out titration calculations, including determining moles of reagents from concentration and volume, identifying mole ratios from balanced equations, and calculating concentration from moles and volume. Several fully worked examples demonstrate how to apply this method to calculate unknown concentrations.giant covalent structures.ppt



giant covalent structures.pptJinsyAjish2
╠²
This document provides an overview of a PowerPoint presentation on structure and bonding for GCSE chemistry students. It introduces ionic bonding, metallic bonding, and covalent bonding. The document explains that the presentation covers how different types of chemical bonding affect the physical properties of elements and compounds. It also provides website information for additional resources on this topic.4341055.ppt



4341055.pptJinsyAjish2
╠²
This document covers ionic compounds and metals. It discusses:
1) How ions form when atoms gain or lose electrons to achieve stable octet configurations, and how ionic bonds form between oppositely charged ions in ionic compounds.
2) Ionic compounds consist of a crystalline lattice of ions with strong electrostatic attractions that give them high melting points and make them brittle and poor conductors.
3) Metals bond through delocalized electrons that form a "sea" of electrons, giving metals malleability, ductility, and high heat and electrical conductivity.7.4 Hess's Law - TE.ppt



7.4 Hess's Law - TE.pptJinsyAjish2
╠²
Hess's law states that the total enthalpy change for a reaction is independent of the pathway taken to get to the final products. It can be used to calculate enthalpy changes that cannot be measured directly through experimentation. The process involves writing balanced equations for each step of the reaction pathway and using standard enthalpy of formation values of reactants and products to determine the enthalpy change. An example is provided where the enthalpy change for the reaction SO2(g) + 2H2S(g) is calculated to be -234.6 kJ/mol by considering it as occurring in two steps and applying Hess's law.seperation techniques.ppt



seperation techniques.pptJinsyAjish2
╠²
This document discusses solutions and solubility. It defines key terms like solute, solvent, and solution. It describes how solubility is affected by temperature. It also explains different techniques for separating mixtures, including filtration, evaporation, distillation, and chromatography.Information-Cards.pptx



Information-Cards.pptxJinsyAjish2
╠²
Graphite is a giant covalent structure made of layers of carbon atoms bonded together, allowing the layers to slide past one another. It conducts electricity and is lubricating. Diamond is also a giant covalent carbon structure, but each carbon atom forms four bonds making it very hard but non-conductive. Buckminster fullerenes are hollow carbon cages about 1 nanometer in diameter, stronger than diamond. Carbon nanotubes are flexible carbon tubes about 1 nanometer wide that conduct electricity and are made from half a buckminster fullerene.Recently uploaded (20)
N.C. DPI's 2023 Language Diversity Briefing



N.C. DPI's 2023 Language Diversity BriefingMebane Rash
╠²
The number of languages spoken in NC public schools.CBSE Arabic Grammar - Class 10 ppt.pptx



CBSE Arabic Grammar - Class 10 ppt.pptxsuhail849886
╠²
cbse arabic grammar
grade 10 cbse arabic grammar
cbse class 10 arabic grammar
arabic marathon cbse arabic 10
nominal sentences
Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
╠²
Prelims of Rass MELAI : a Music, Entertainment, Literature, Arts and Internet Culture Quiz organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. Eng7-Q4-Lesson 1 Part 1 Understanding Discipline-Specific Words, Voice, and T...



Eng7-Q4-Lesson 1 Part 1 Understanding Discipline-Specific Words, Voice, and T...sandynavergas1
╠²
Understanding Discipline-Specific Words, Voice, and Technical TermsAPM People Interest Network Conference - Tim Lyons - The neurological levels ...



APM People Interest Network Conference - Tim Lyons - The neurological levels ...Association for Project Management
╠²
APM People Interest Network Conference 2025
-Autonomy, Teams and Tension: Projects under stress
-Tim Lyons
-The neurological levels of
team-working: Harmony and tensions
With a background in projects spanning more than 40 years, Tim Lyons specialised in the delivery of large, complex, multi-disciplinary programmes for clients including Crossrail, Network Rail, ExxonMobil, Siemens and in patent development. His first career was in broadcasting, where he designed and built commercial radio station studios in Manchester, Cardiff and Bristol, also working as a presenter and programme producer. Tim now writes and presents extensively on matters relating to the human and neurological aspects of projects, including communication, ethics and coaching. He holds a MasterŌĆÖs degree in NLP, is an NLP Master Practitioner and International Coach. He is the Deputy Lead for APMŌĆÖs People Interest Network.
Session | The Neurological Levels of Team-working: Harmony and Tensions
Understanding how teams really work at conscious and unconscious levels is critical to a harmonious workplace. This session uncovers what those levels are, how to use them to detect and avoid tensions and how to smooth the management of change by checking you have considered all of them.Reordering Rules in Odoo 17 Inventory - Odoo ║▌║▌▀Żs



Reordering Rules in Odoo 17 Inventory - Odoo ║▌║▌▀ŻsCeline George
╠²
In Odoo 17, the Inventory module allows us to set up reordering rules to ensure that our stock levels are maintained, preventing stockouts. Let's explore how this feature works.Computer Network Unit IV - Lecture Notes - Network Layer



Computer Network Unit IV - Lecture Notes - Network LayerMurugan146644
╠²
Title:
Lecture Notes - Unit IV - The Network Layer
Description:
Welcome to the comprehensive guide on Computer Network concepts, tailored for final year B.Sc. Computer Science students affiliated with Alagappa University. This document covers fundamental principles and advanced topics in Computer Network. PDF content is prepared from the text book Computer Network by Andrew S. Tenanbaum
Key Topics Covered:
Main Topic : The Network Layer
Sub-Topic : Network Layer Design Issues (Store and forward packet switching , service provided to the transport layer, implementation of connection less service, implementation of connection oriented service, Comparision of virtual circuit and datagram subnet), Routing algorithms (Shortest path routing, Flooding , Distance Vector routing algorithm, Link state routing algorithm , hierarchical routing algorithm, broadcast routing, multicast routing algorithm)
Other Link :
1.Introduction to computer network - /slideshow/lecture-notes-introduction-to-computer-network/274183454
2. Physical Layer - /slideshow/lecture-notes-unit-ii-the-physical-layer/274747125
3. Data Link Layer Part 1 : /slideshow/lecture-notes-unit-iii-the-datalink-layer/275288798
Target Audience:
Final year B.Sc. Computer Science students at Alagappa University seeking a solid foundation in Computer Network principles for academic.
About the Author:
Dr. S. Murugan is Associate Professor at Alagappa Government Arts College, Karaikudi. With 23 years of teaching experience in the field of Computer Science, Dr. S. Murugan has a passion for simplifying complex concepts in Computer Network
Disclaimer:
This document is intended for educational purposes only. The content presented here reflects the authorŌĆÖs understanding in the field of Computer NetworkHow to Setup WhatsApp in Odoo 17 - Odoo ║▌║▌▀Żs



How to Setup WhatsApp in Odoo 17 - Odoo ║▌║▌▀ŻsCeline George
╠²
Integrate WhatsApp into Odoo using the WhatsApp Business API or third-party modules to enhance communication. This integration enables automated messaging and customer interaction management within Odoo 17.Adventure Activities Final By H R Gohil Sir



Adventure Activities Final By H R Gohil SirGUJARATCOMMERCECOLLE
╠²
Adventure Activities Final By H R Gohil SirDigital Tools with AI for e-Content Development.pptx



Digital Tools with AI for e-Content Development.pptxDr. Sarita Anand
╠²
This ppt is useful for not only for B.Ed., M.Ed., M.A. (Education) or any other PG level students or Ph.D. scholars but also for the school, college and university teachers who are interested to prepare an e-content with AI for their students and others.Database population in Odoo 18 - Odoo slides



Database population in Odoo 18 - Odoo slidesCeline George
╠²
In this slide, weŌĆÖll discuss the database population in Odoo 18. In Odoo, performance analysis of the source code is more important. Database population is one of the methods used to analyze the performance of our code. Kaun TALHA quiz Prelims - El Dorado 2025



Kaun TALHA quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
╠²
Prelims of Kaun TALHA : a Travel, Architecture, Lifestyle, Heritage and Activism quiz, organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. Essentials of a Good PMO, presented by Aalok Sonawala



Essentials of a Good PMO, presented by Aalok SonawalaAssociation for Project Management
╠²
APM event hosted by the South Wales and West of England Network (SWWE Network)
Speaker: Aalok Sonawala
The SWWE Regional Network were very pleased to welcome Aalok Sonawala, Head of PMO, National Programmes, Rider Levett Bucknall on 26 February, to BAWA for our first face to face event of 2025. Aalok is a member of APMŌĆÖs Thames Valley Regional Network and also speaks to members of APMŌĆÖs PMO Interest Network, which aims to facilitate collaboration and learning, offer unbiased advice and guidance.
Tonight, Aalok planned to discuss the importance of a PMO within project-based organisations, the different types of PMO and their key elements, PMO governance and centres of excellence.
PMOŌĆÖs within an organisation can be centralised, hub and spoke with a central PMO with satellite PMOs globally, or embedded within projects. The appropriate structure will be determined by the specific business needs of the organisation. The PMO sits above PM delivery and the supply chain delivery teams.
For further information about the event please click here.South Hornsey: The Lost Local Authority that Merged with Stoke Newington by T...



South Hornsey: The Lost Local Authority that Merged with Stoke Newington by T...History of Stoke Newington
╠²
Presented at the 24th Stoke Newington History Talks event on 27th Feb 2025
https://stokenewingtonhistory.com/stoke-newington-history-talks/QuickBooks Desktop to QuickBooks Online How to Make the Move



QuickBooks Desktop to QuickBooks Online How to Make the MoveTechSoup
╠²
If you use QuickBooks Desktop and are stressing about moving to QuickBooks Online, in this webinar, get your questions answered and learn tips and tricks to make the process easier for you.
Key Questions:
* When is the best time to make the shift to QuickBooks Online?
* Will my current version of QuickBooks Desktop stop working?
* I have a really old version of QuickBooks. What should I do?
* I run my payroll in QuickBooks Desktop now. How is that affected?
*Does it bring over all my historical data? Are there things that don't come over?
* What are the main differences between QuickBooks Desktop and QuickBooks Online?
* And moreRass MELAI : an Internet MELA Quiz Finals - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
╠²
Finals of Rass MELAI : a Music, Entertainment, Literature, Arts and Internet Culture Quiz organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. A PPT Presentation on The Princess and the God: A tale of ancient India by A...



A PPT Presentation on The Princess and the God: A tale of ancient India by A...Beena E S
╠²
A PPT Presentation on The Princess and the God: A tale of ancient India by Aaron ShepardRass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
╠²
APM People Interest Network Conference - Tim Lyons - The neurological levels ...



APM People Interest Network Conference - Tim Lyons - The neurological levels ...Association for Project Management
╠²
Kaun TALHA quiz Prelims - El Dorado 2025



Kaun TALHA quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
╠²
South Hornsey: The Lost Local Authority that Merged with Stoke Newington by T...



South Hornsey: The Lost Local Authority that Merged with Stoke Newington by T...History of Stoke Newington
╠²
Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
╠²
ionic equation and net ionic equation PPT
- 2. Net-ionic Equations ’üĮ Net ionic equations are useful in that they show only those chemical species directly participating in a chemical reaction ’üĮ The keys to being able to write net ionic equations are the ability to recognize monatomic and polyatomic ions, the solubility rules, and the rules for electrolyte behavior.
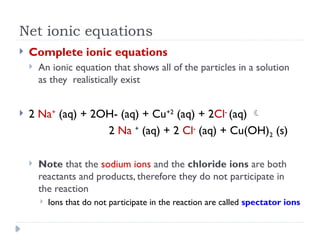
- 3. Net ionic equations ’üĮ Complete ionic equations ’üĮ An ionic equation that shows all of the particles in a solution as they realistically exist ’üĮ 2 Na+ (aq) + 2OH- (aq) + Cu+2 (aq) + 2Cl- (aq) ’āĀ 2 Na + (aq) + 2 Cl- (aq) + Cu(OH)2 (s) ’üĮ Note that the sodium ions and the chloride ions are both reactants and products, therefore they do not participate in the reaction ’üĮ Ions that do not participate in the reaction are called spectator ions
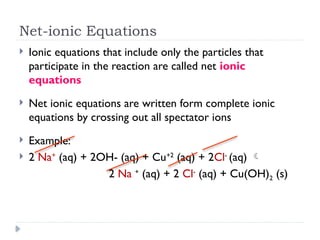
- 4. Net-ionic Equations ’üĮ Ionic equations that include only the particles that participate in the reaction are called net ionic equations ’üĮ Net ionic equations are written form complete ionic equations by crossing out all spectator ions ’üĮ Example: ’üĮ 2 Na+ (aq) + 2OH- (aq) + Cu+2 (aq) + 2Cl- (aq) ’āĀ 2 Na + (aq) + 2 Cl- (aq) + Cu(OH)2 (s)
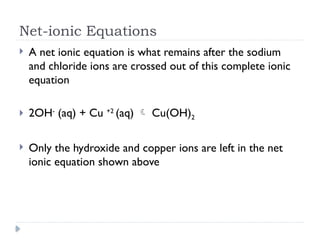
- 5. Net-ionic Equations ’üĮ A net ionic equation is what remains after the sodium and chloride ions are crossed out of this complete ionic equation ’üĮ 2OH- (aq) + Cu +2 (aq) ’āĀ Cu(OH)2 ’üĮ Only the hydroxide and copper ions are left in the net ionic equation shown above
- 6. Example: ’üĮ Write the chemical, complete ionic, and net ionic equations for the rxn between aqueous solutions of barium nitrate and sodium carbonate that forms the precipitate barium carbonate. ’üĮ Complete Ionic Equation: ’üĮ Ba(NO3)2 (aq) + Na2CO3 (aq) ’āĀ BaCO3 (s) + NaNO3 (aq) ’üĮ Balance the equation above: ’üĮ Ba(NO3)2(aq) + Na2CO3 (aq) + BaCO3 (s) + 2 NaNO3 (aq)
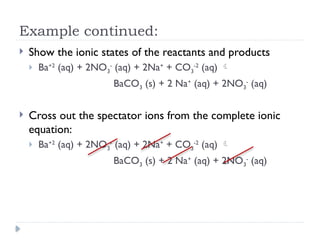
- 7. Example continued: ’üĮ Show the ionic states of the reactants and products ’üĮ Ba+2 (aq) + 2NO3 - (aq) + 2Na+ + CO3 -2 (aq) ’āĀ BaCO3 (s) + 2 Na+ (aq) + 2NO3 - (aq) ’üĮ Cross out the spectator ions from the complete ionic equation: ’üĮ Ba+2 (aq) + 2NO3 - (aq) + 2Na+ + CO3 -2 (aq) ’āĀ BaCO3 (s) + 2 Na+ (aq) + 2NO3 - (aq)
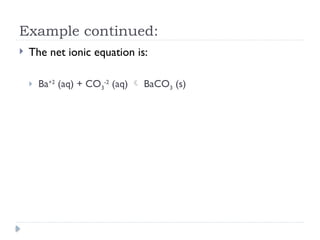
- 8. Example continued: ’üĮ The net ionic equation is: ’üĮ Ba+2 (aq) + CO3 -2 (aq) ’āĀ BaCO3 (s)








