1 of 2
Download to read offline

Recommended
BÃ i Phenol



BÃ i PhenolKim NgÃĒn
Ėý
BÃ i 41: PHENOL
Bà i bao gáŧm cášĨu tᚥo phÃĒn táŧ cáŧ§a phenol, tÃnh chášĨt hÃģa háŧc cáŧ§a phenol, cÃĄc áŧĐng dáŧĨng cáŧ§a phenol trong Äáŧi sáŧng và bà i tášp cÃģ láŧi giášĢi.Phenol



PhenolKamariah Abdullah Joanna Libin
Ėý
Phenols are chemical compounds that contain a hydroxyl group attached to an aromatic hydrocarbon group. Phenols are hydroxyl derivatives of hydrocarbons where a hydrogen on the benzene ring is replaced by a hydroxyl group. Phenols react in various ways including forming salts with bases, undergoing oxidation, and reacting with bromine water, nitric acid, iron chloride, and other compounds. Phenols have medical uses as keratolytics, antipruritics, and disinfectants due to their caustic effects on tissues.Phenol



PhenolSummervir Cheema
Ėý
This document discusses methods for removing phenol contaminants like bisphenol A (BPA) from water samples. It explores using the enzyme polyphenol oxidase (PPO) found in plants to oxidize phenols into colored quinones, which are then captured by chitosan. Specifically, potato extract is shown to effectively oxidize BPA as monitored by UV-Vis spectroscopy. Optimization of variables like potato extract concentration, chitosan amount, and reaction conditions is discussed. Magnetic chitosan beads are also prepared and tested for removing oxidized BPA products, demonstrating an approach for removing phenol contaminants from the environment.P H E N O L S Report



P H E N O L S Reportaldebaran4
Ėý
Phenols are a class of chemical compounds consisting of a hydroxyl group bonded directly to an aromatic hydrocarbon group. The simplest phenol is phenol itself, which has a chemical formula of C6H5OH and a structure of a hydroxyl group bonded to a phenyl ring. Phenols have higher acidities than alcohols due to the aromatic ring's tight coupling with the oxygen and a loose bond between oxygen and hydrogen. Phenols are found naturally, especially in plants, and are also used industrially and in various medicinals like aspirin and propofol.TrÃē ChÆĄi : VÆ°ÆĄng Miáŧn HÃģa háŧc



TrÃē ChÆĄi : VÆ°ÆĄng Miáŧn HÃģa háŧcNguyenDinh Phuoc
Ėý
LUᚎT CHÆ I :
Láŧp chia là m 2 Äáŧi: Äáŧi A và Äáŧi B. Máŧi Äáŧi sáš― lᚧn lÆ°áŧĢt xoay vÃēng trÃēn vÆ°ÆĄng miáŧn váŧi 5 lÄĐnh váŧąc khÃĄc nhau cÃđng 1 Ãī may mášŊn(vÆ°ÆĄng miáŧn và ng) và 1 Ãī xui xášŧo(vÆ°ÆĄng miáŧn Äen). áŧĻng váŧi máŧi lÄĐnh váŧąc, sáš― cÃģ máŧt báŧ gáŧm 4 cÃĒu háŧi liÊn quan Äášŋn lÄĐnh váŧąc ÄÃģ. Äáŧi nà o trášĢ láŧi sai thÃŽ mášĨt lÆ°áŧĢt, ÄÚng thÃŽ trášĢ láŧi tiášŋp. Äáŧi nà o trášĢ láŧi ÄÚng 2 cÃĒu liÊn tiášŋp áŧ bášĨt cáŧĐ lÄĐnh váŧąc nà o sáš― ÄÆ°áŧĢc 1 vÆ°ÆĄng miáŧn.
Nášŋu xoay và o ÄÚng Ãī may mášŊn, Äáŧi sáš― ÄÆ°áŧĢc cháŧn lÄĐnh váŧąc sáŧ trÆ°áŧng nhášĨt tÃđy Ã―, cÃēn nášŋu và o Ãī xui xášŧo, sáŧą láŧąa cháŧn lÄĐnh váŧąc sáš― rÆĄi và o Äáŧi cÃēn lᚥi(lÆ°u Ã―:k phášĢi mášĨt lÆ°áŧĢt, mà là Äáŧi phÆ°ÆĄng cháŧn lÄĐnh váŧąc cho mÃŽnh).
TrÃē chÆĄi sáš― dáŧŦng khi Äáŧi nà o cÃģ 5 vÆ°ÆĄng miáŧn trÆ°áŧc, hoáš·c khi cášĢ 2 Äáŧi chÆ°a Äᚥt ÄÆ°áŧĢc 5 vÆ°ÆĄng miáŧn mà ÄÃĢ hášŋt gÃģi cÃĒu háŧi thÃŽ sáš― so sÃĄnh sáŧ vÆ°ÆĄng miáŧn Äᚥt ÄÆ°áŧĢc, nášŋu 2 Äáŧi ngang nhau, Äiáŧm sáŧ sáš― pháŧĨ thuáŧc và o tinh thᚧn hÄng hÃĄi, Äoà n kášŋt cáŧ§a máŧi Äáŧi thÃīng qua cÃĄc giÃĄm khášĢo cuáŧc thi ÄÃĄnh giÃĄ !PPt háŧĢp chášĨt cÃģ oxi cáŧ§a lÆ°u huáŧģnh ( tiášŋt 2: Axit sunfuric)



PPt háŧĢp chášĨt cÃģ oxi cáŧ§a lÆ°u huáŧģnh ( tiášŋt 2: Axit sunfuric)thuyan232
Ėý
HáŧĢp chášĨt cÃģ oxi cáŧ§a lÆ°u huáŧģnh (tiášŋt 2: Axit sunfuric)Ãī CháŧŊ hÃģa háŧc



Ãī CháŧŊ hÃģa háŧcHoa Chà LÃ
Ėý
ÄÃĒy là phᚧn trÃē chÆĄi, cÃĄc bᚥn xem và like giÚp mÃŽnh nhÃĐ.ALCOHOL and PHENOL



ALCOHOL and PHENOLGladys Jane Franken
Ėý
Alcohols contain an -OH group bonded to a carbon atom. They are classified based on the carbon the OH group is attached to as primary, secondary, or tertiary alcohols. Alcohols have higher boiling points than similar hydrocarbons due to hydrogen bonding. Common alcohols include methanol, ethanol, and isopropyl alcohol. Alcohols are used in drinks, fuels, solvents, and to synthesize other organic compounds. Phenol contains an OH group bonded directly to a benzene ring. It is used as an antiseptic and in making resins, plastics, and pharmaceuticals. Phenol undergoes electrophilic aromatic substitution reactions more readily than benzPhenolic compounds



Phenolic compoundsZuby Gohar Ansari
Ėý
The document discusses plant phenolic compounds. It notes that thousands of phenolic structures are known, accounting for 40% of organic carbon in the biosphere. Phenolic compounds are primarily derived from the phenylpropanoid and acetate pathways and play important roles in plant cell walls, defense, wood/bark features, and flower color/flavor. Phenolic compounds can be classified into flavonoids and non-flavonoids. Key flavonoids discussed include quercetin, naringenin, and isoflavonoids like genistein and daidzein. Non-flavonoids include hydroxycinnamates, stilbenes like resveratrol, and tannins.Phenols



PhenolsNove Joy DeleÃąa
Ėý
Phenol and its derivatives have both IUPAC and common names. Phenol is a white crystalline solid that is hazardous to skin. It has higher melting and boiling points than toluene due to hydrogen bonding between phenol molecules. Phenol is moderately soluble in water and weakly acidic. Major uses of phenol involve its conversion to plastics, resins, nylon precursors, and drugs like aspirin. It is also used as a throat pain reliever and in nucleic acid extractions.Btl2 vatlieupolime



Btl2 vatlieupolimeKháŧ ÄÃt Äáŧ
Ėý
BÃ i tášp láŧn 2
Thiášŋt kášŋ bà i giášĢng Äiáŧn táŧ
Láŧp 12 NC
ChÆ°ÆĄng IV: Polime va vášt liáŧu polime
Bà i 17: Vášt liáŧu PolimeKbsp trÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥi



Kbsp trÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥiKháŧ ÄÃt Äáŧ
Ėý
Káŧch bášĢn sÆ° phᚥm
TrÃē chÆĄi Ãī cháŧŊ - Äᚥi CÆ°ÆĄng Váŧ Kim LoᚥiVbhd trÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥi



Vbhd trÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥiKháŧ ÄÃt Äáŧ
Ėý
VÄn BášĢn hÆ°áŧng dášŦn tháŧąc hiáŧn
TrÃē chÆĄi Ãī cháŧŊ - Äᚥi CÆ°ÆĄng Váŧ Kim LoᚥiTrÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥi



TrÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥiKháŧ ÄÃt Äáŧ
Ėý
TrÃē chÆĄi Ãī cháŧŊ - Äᚥi CÆ°ÆĄng váŧ Kim LoᚥiVbhd trÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥi



Vbhd trÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥiKháŧ ÄÃt Äáŧ
Ėý
VÄn bášĢn hÆ°áŧng dášŦn tháŧąc hiáŧn
TrÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥi.More Related Content
Viewers also liked (16)
Phenol



PhenolSummervir Cheema
Ėý
This document discusses methods for removing phenol contaminants like bisphenol A (BPA) from water samples. It explores using the enzyme polyphenol oxidase (PPO) found in plants to oxidize phenols into colored quinones, which are then captured by chitosan. Specifically, potato extract is shown to effectively oxidize BPA as monitored by UV-Vis spectroscopy. Optimization of variables like potato extract concentration, chitosan amount, and reaction conditions is discussed. Magnetic chitosan beads are also prepared and tested for removing oxidized BPA products, demonstrating an approach for removing phenol contaminants from the environment.P H E N O L S Report



P H E N O L S Reportaldebaran4
Ėý
Phenols are a class of chemical compounds consisting of a hydroxyl group bonded directly to an aromatic hydrocarbon group. The simplest phenol is phenol itself, which has a chemical formula of C6H5OH and a structure of a hydroxyl group bonded to a phenyl ring. Phenols have higher acidities than alcohols due to the aromatic ring's tight coupling with the oxygen and a loose bond between oxygen and hydrogen. Phenols are found naturally, especially in plants, and are also used industrially and in various medicinals like aspirin and propofol.TrÃē ChÆĄi : VÆ°ÆĄng Miáŧn HÃģa háŧc



TrÃē ChÆĄi : VÆ°ÆĄng Miáŧn HÃģa háŧcNguyenDinh Phuoc
Ėý
LUᚎT CHÆ I :
Láŧp chia là m 2 Äáŧi: Äáŧi A và Äáŧi B. Máŧi Äáŧi sáš― lᚧn lÆ°áŧĢt xoay vÃēng trÃēn vÆ°ÆĄng miáŧn váŧi 5 lÄĐnh váŧąc khÃĄc nhau cÃđng 1 Ãī may mášŊn(vÆ°ÆĄng miáŧn và ng) và 1 Ãī xui xášŧo(vÆ°ÆĄng miáŧn Äen). áŧĻng váŧi máŧi lÄĐnh váŧąc, sáš― cÃģ máŧt báŧ gáŧm 4 cÃĒu háŧi liÊn quan Äášŋn lÄĐnh váŧąc ÄÃģ. Äáŧi nà o trášĢ láŧi sai thÃŽ mášĨt lÆ°áŧĢt, ÄÚng thÃŽ trášĢ láŧi tiášŋp. Äáŧi nà o trášĢ láŧi ÄÚng 2 cÃĒu liÊn tiášŋp áŧ bášĨt cáŧĐ lÄĐnh váŧąc nà o sáš― ÄÆ°áŧĢc 1 vÆ°ÆĄng miáŧn.
Nášŋu xoay và o ÄÚng Ãī may mášŊn, Äáŧi sáš― ÄÆ°áŧĢc cháŧn lÄĐnh váŧąc sáŧ trÆ°áŧng nhášĨt tÃđy Ã―, cÃēn nášŋu và o Ãī xui xášŧo, sáŧą láŧąa cháŧn lÄĐnh váŧąc sáš― rÆĄi và o Äáŧi cÃēn lᚥi(lÆ°u Ã―:k phášĢi mášĨt lÆ°áŧĢt, mà là Äáŧi phÆ°ÆĄng cháŧn lÄĐnh váŧąc cho mÃŽnh).
TrÃē chÆĄi sáš― dáŧŦng khi Äáŧi nà o cÃģ 5 vÆ°ÆĄng miáŧn trÆ°áŧc, hoáš·c khi cášĢ 2 Äáŧi chÆ°a Äᚥt ÄÆ°áŧĢc 5 vÆ°ÆĄng miáŧn mà ÄÃĢ hášŋt gÃģi cÃĒu háŧi thÃŽ sáš― so sÃĄnh sáŧ vÆ°ÆĄng miáŧn Äᚥt ÄÆ°áŧĢc, nášŋu 2 Äáŧi ngang nhau, Äiáŧm sáŧ sáš― pháŧĨ thuáŧc và o tinh thᚧn hÄng hÃĄi, Äoà n kášŋt cáŧ§a máŧi Äáŧi thÃīng qua cÃĄc giÃĄm khášĢo cuáŧc thi ÄÃĄnh giÃĄ !PPt háŧĢp chášĨt cÃģ oxi cáŧ§a lÆ°u huáŧģnh ( tiášŋt 2: Axit sunfuric)



PPt háŧĢp chášĨt cÃģ oxi cáŧ§a lÆ°u huáŧģnh ( tiášŋt 2: Axit sunfuric)thuyan232
Ėý
HáŧĢp chášĨt cÃģ oxi cáŧ§a lÆ°u huáŧģnh (tiášŋt 2: Axit sunfuric)Ãī CháŧŊ hÃģa háŧc



Ãī CháŧŊ hÃģa háŧcHoa Chà LÃ
Ėý
ÄÃĒy là phᚧn trÃē chÆĄi, cÃĄc bᚥn xem và like giÚp mÃŽnh nhÃĐ.ALCOHOL and PHENOL



ALCOHOL and PHENOLGladys Jane Franken
Ėý
Alcohols contain an -OH group bonded to a carbon atom. They are classified based on the carbon the OH group is attached to as primary, secondary, or tertiary alcohols. Alcohols have higher boiling points than similar hydrocarbons due to hydrogen bonding. Common alcohols include methanol, ethanol, and isopropyl alcohol. Alcohols are used in drinks, fuels, solvents, and to synthesize other organic compounds. Phenol contains an OH group bonded directly to a benzene ring. It is used as an antiseptic and in making resins, plastics, and pharmaceuticals. Phenol undergoes electrophilic aromatic substitution reactions more readily than benzPhenolic compounds



Phenolic compoundsZuby Gohar Ansari
Ėý
The document discusses plant phenolic compounds. It notes that thousands of phenolic structures are known, accounting for 40% of organic carbon in the biosphere. Phenolic compounds are primarily derived from the phenylpropanoid and acetate pathways and play important roles in plant cell walls, defense, wood/bark features, and flower color/flavor. Phenolic compounds can be classified into flavonoids and non-flavonoids. Key flavonoids discussed include quercetin, naringenin, and isoflavonoids like genistein and daidzein. Non-flavonoids include hydroxycinnamates, stilbenes like resveratrol, and tannins.Phenols



PhenolsNove Joy DeleÃąa
Ėý
Phenol and its derivatives have both IUPAC and common names. Phenol is a white crystalline solid that is hazardous to skin. It has higher melting and boiling points than toluene due to hydrogen bonding between phenol molecules. Phenol is moderately soluble in water and weakly acidic. Major uses of phenol involve its conversion to plastics, resins, nylon precursors, and drugs like aspirin. It is also used as a throat pain reliever and in nucleic acid extractions.More from Kháŧ ÄÃt Äáŧ (7)
Btl2 vatlieupolime



Btl2 vatlieupolimeKháŧ ÄÃt Äáŧ
Ėý
BÃ i tášp láŧn 2
Thiášŋt kášŋ bà i giášĢng Äiáŧn táŧ
Láŧp 12 NC
ChÆ°ÆĄng IV: Polime va vášt liáŧu polime
Bà i 17: Vášt liáŧu PolimeKbsp trÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥi



Kbsp trÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥiKháŧ ÄÃt Äáŧ
Ėý
Káŧch bášĢn sÆ° phᚥm
TrÃē chÆĄi Ãī cháŧŊ - Äᚥi CÆ°ÆĄng Váŧ Kim LoᚥiVbhd trÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥi



Vbhd trÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥiKháŧ ÄÃt Äáŧ
Ėý
VÄn BášĢn hÆ°áŧng dášŦn tháŧąc hiáŧn
TrÃē chÆĄi Ãī cháŧŊ - Äᚥi CÆ°ÆĄng Váŧ Kim LoᚥiTrÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥi



TrÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥiKháŧ ÄÃt Äáŧ
Ėý
TrÃē chÆĄi Ãī cháŧŊ - Äᚥi CÆ°ÆĄng váŧ Kim LoᚥiVbhd trÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥi



Vbhd trÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥiKháŧ ÄÃt Äáŧ
Ėý
VÄn bášĢn hÆ°áŧng dášŦn tháŧąc hiáŧn
TrÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥi.TrÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥi



TrÃē chÆĄi Ãī cháŧŊ Äᚥi cÆ°ÆĄng kim loᚥiKháŧ ÄÃt Äáŧ
Ėý
TrÃē chÆĄi Ãī cháŧŊ, ÃĄp dáŧĨng trong tiášŋt Ãīn tášp chÆ°ÆĄng Äᚥi CÆ°ÆĄng váŧ kim loᚥi, chÆ°ÆĄng trinh láŧp 12, ban cÆĄ bášĢnRecently uploaded (18)
[PPT11] Bà i 7 - Äáŧc - Cà Mau quÊ xáŧĐ.pptx![[PPT11] Bà i 7 - Äáŧc - Cà Mau quÊ xáŧĐ.pptx](https://cdn.slidesharecdn.com/ss_thumbnails/ppt11bi7-c-cmauqux-250305070313-62766c8d-thumbnail.jpg?width=560&fit=bounds)
![[PPT11] Bà i 7 - Äáŧc - Cà Mau quÊ xáŧĐ.pptx](https://cdn.slidesharecdn.com/ss_thumbnails/ppt11bi7-c-cmauqux-250305070313-62766c8d-thumbnail.jpg?width=560&fit=bounds)
![[PPT11] Bà i 7 - Äáŧc - Cà Mau quÊ xáŧĐ.pptx](https://cdn.slidesharecdn.com/ss_thumbnails/ppt11bi7-c-cmauqux-250305070313-62766c8d-thumbnail.jpg?width=560&fit=bounds)
![[PPT11] Bà i 7 - Äáŧc - Cà Mau quÊ xáŧĐ.pptx](https://cdn.slidesharecdn.com/ss_thumbnails/ppt11bi7-c-cmauqux-250305070313-62766c8d-thumbnail.jpg?width=560&fit=bounds)
[PPT11] Bà i 7 - Äáŧc - Cà Mau quÊ xáŧĐ.pptxphuonguyn2400
Ėý
TÃŽm hiáŧu vÄn bášĢn cà mau quÊ xáŧĐ- ngáŧŊ vÄn 11[PPT11] Bà i 7 - Äáŧc - Và tÃīi vášŦn muáŧn mášđ....ppt![[PPT11] Bà i 7 - Äáŧc - Và tÃīi vášŦn muáŧn mášđ....ppt](https://cdn.slidesharecdn.com/ss_thumbnails/ppt11bi7-c-vtivnmunm-250305063122-6e221a47-thumbnail.jpg?width=560&fit=bounds)
![[PPT11] BÃ i 7 - Äáŧc - VÃ tÃīi vášŦn muáŧn mášđ....ppt](https://cdn.slidesharecdn.com/ss_thumbnails/ppt11bi7-c-vtivnmunm-250305063122-6e221a47-thumbnail.jpg?width=560&fit=bounds)
![[PPT11] BÃ i 7 - Äáŧc - VÃ tÃīi vášŦn muáŧn mášđ....ppt](https://cdn.slidesharecdn.com/ss_thumbnails/ppt11bi7-c-vtivnmunm-250305063122-6e221a47-thumbnail.jpg?width=560&fit=bounds)
![[PPT11] BÃ i 7 - Äáŧc - VÃ tÃīi vášŦn muáŧn mášđ....ppt](https://cdn.slidesharecdn.com/ss_thumbnails/ppt11bi7-c-vtivnmunm-250305063122-6e221a47-thumbnail.jpg?width=560&fit=bounds)
[PPT11] BÃ i 7 - Äáŧc - VÃ tÃīi vášŦn muáŧn mášđ....pptphuonguyn2400
Ėý
VÃ tÃīi vášŦn muáŧn mášđ - NgáŧŊ vÄn 11ChÆ°ÆĄng 3. Äáŧi lÆ°u nhiáŧt. hÃģa háŧŊu cÆĄ TDTU



ChÆ°ÆĄng 3. Äáŧi lÆ°u nhiáŧt. hÃģa háŧŊu cÆĄ TDTUngKhi80
Ėý
tà i liáŧu tham khášĢo hÃģa háŧŊu cÆĄ slideCHINH PHáŧĪC Là THUYášūT SINH HáŧC Báš°NG SÆ Äáŧ TÆŊ DUY.pdf



CHINH PHáŧĪC LÃ THUYášūT SINH HáŧC Báš°NG SÆ Äáŧ TÆŊ DUY.pdfHuyn804581
Ėý
LÃ― thuyášŋt 12 bášąng sÆĄ Äáŧ tÆ° duy dáŧ
nháŧ, dáŧ
hiáŧu háŧ tháŧngcd-van-6_-t47-b4-thtv-tu-dong-am-tu-da-nghia_11072023.pptx



cd-van-6_-t47-b4-thtv-tu-dong-am-tu-da-nghia_11072023.pptxThyLinh936093
Ėý
bà i tášp tiášŋng viáŧt váŧ táŧŦ Äáŧng ÃĒm táŧŦ Äa nghÄĐa NghiÊn cáŧĐu sinh háŧc váŧ Äáŧt biášŋn Nhiáŧ
m sášŊc tháŧ



NghiÊn cáŧĐu sinh háŧc váŧ Äáŧt biášŋn Nhiáŧ
m sášŊc tháŧnguyenphuonguyen1412
Ėý
Äáŧt biášŋn cášĨu trÚc và Äáŧt biášŋn sáŧ lÆ°áŧĢng NSTMICE TrÆ°áŧng Anh ngáŧŊ IU Cebu Brochure 2025.pdf



MICE TrÆ°áŧng Anh ngáŧŊ IU Cebu Brochure 2025.pdfDu háŧc MICE - Du háŧc tiášŋng Anh
Ėý
https://tienganhtaiphi.com/truong-anh-ngu-iu-cebu/
IU English Academy cam kášŋt mang Äášŋn máŧt chÆ°ÆĄng trÃŽnh háŧc tiášŋng Anh toà n diáŧn dà nh cho háŧc viÊn chuášĐn báŧ du háŧc hoáš·c là m viáŧc quáŧc tášŋ. BÊn cᚥnh viáŧc rÃĻn luyáŧn ngÃīn ngáŧŊ, IU Äáš·c biáŧt chÚ tráŧng Äášŋn cÃĄc hoᚥt Äáŧng ngoᚥi khÃģa, bao gáŧm cÃĄc láŧp tháŧ thao fitness nhÆ° Yoga, Kickboxing và nhášĢy Zumba. NháŧŊng hoᚥt Äáŧng nà y khÃīng cháŧ nÃĒng cao sáŧĐc kháŧe mà cÃēn giÚp phÃĄt triáŧn káŧđ nÄng là m viáŧc nhÃģm và tinh thᚧn tháŧ thao, giÚp háŧc viÊn sášĩn sà ng cho máŧi tháŧ thÃĄch trong tÆ°ÆĄng lai.
==== Du háŧc MICE - Du háŧc tiášŋng Anh ====
ðĄ CÃīng ty TNHH tÆ° vášĨn MICE
ðą Hotline/Zalo/Viber: 0904137471
ð§ info@tienganhtaiphi.com
ðąïļ http://tienganhtaiphi.com
ðąïļ NhÃģm háŧc TA online 1 kÃĻm 1: https://www.facebook.com/groups/2157125567720037
ðŽ 39/15 ÄÆ°áŧng 102, P. TÄng NhÆĄn PhÚ A, TP. Tháŧ§ ÄáŧĐc (Q9), TP.HCM
Phenol
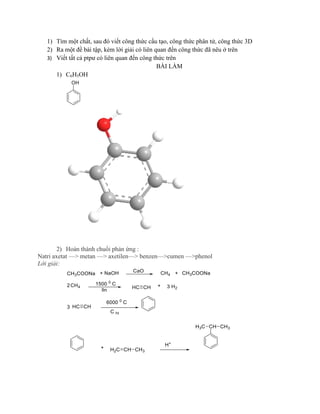
- 1. 1) TÃŽm máŧt chášĨt, sau ÄÃģ viášŋt cÃīng tháŧĐc cášĨu tᚥo, cÃīng tháŧĐc phÃĒn táŧ, cÃīng tháŧĐc 3D 2) Ra máŧt Äáŧ bà i tášp, kÃĻm láŧi giášĢi cÃģ liÊn quan Äášŋn cÃīng tháŧĐc ÄÃĢ nÊu áŧ trÊn 3) Viášŋt tášĨt cášĢ ptpÆ° cÃģ liÊn quan Äášŋn cÃīng tháŧĐc trÊn BÃI LÃM 1) C6H5OH 2) Hoà n thà nh chuáŧi phášĢn áŧĐng : Natri axetat â> metan â> axetilenâ> benzenâ>cumen â>phenol Láŧi giášĢi:
- 2. 3) - tÃĄc dáŧĨng váŧi dd Br2: - TÃĄc dáŧĨng váŧi kim loᚥi kiáŧm: - TÃĄc dáŧĨng váŧi bazÆĄ:


























