potentiometric titration ^.pptx
Download as PPTX, PDF0 likes2,222 views
This document discusses potentiometric titration, which is a technique that measures the potential between two electrodes to determine the concentration of a solute. It involves using a reference electrode, salt bridge, analyte solution, and indicator electrode in an electrochemical cell. The potential difference is measured as the titrant is added and the concentration of ions changes. There are several types of potentiometric titrations including acid-base, redox, complexometric, and precipitation titrations. The principle involves measuring the potential difference created by the indicator and reference electrodes in response to changes in the analyte solution during titration.
1 of 13
Download to read offline










Recommended
Polarography Principle, instrumentation, Applications.pptx



Polarography Principle, instrumentation, Applications.pptxVandana Devesh Sharma
Ěý
Polarography is an electroanalytical technique that uses a dropping mercury electrode (DME) and measures the current between two electrodes when a gradually increasing voltage is applied. The current-voltage curve obtained is used to determine analyte concentration from the diffusion current and identify species from the characteristic half-wave potential. The Ilkovic equation relates diffusion current to analyte properties like concentration, number of electrons involved, and diffusion coefficient. Polarography finds applications in qualitative and quantitative analysis of metals, drugs, and organic compounds.Conductometry



ConductometryAsmitaSatao
Ěý
Conductometry is a technique that measures the electrical conductivity of a solution during a chemical reaction or titration. It works on the principle that conductivity changes as ions are replaced by other ions. The instrumentation includes a conductivity cell with electrodes, a current source, and a conductivity meter. Conductometry has applications in determining water quality, solubility of salts, and as an analytical technique for titrations. It provides accurate results but is limited for some redox titrations where hydronium ion concentration masks conductivity changes.Polarography principle and instrumentation



Polarography principle and instrumentationKIRANBARBATKAR
Ěý
Jaroslav Heyrovsky invented polarography in 1922 and won the Nobel Prize for it in 1959. Polarography involves using a dropping mercury electrode (DME) and saturated calomel electrode (SCE) to study the electrical properties of solutions through electrolysis. As mercury drops from the DME into the solution, the current is measured at different voltages to generate a polarogram curve and determine the concentration and nature of solutes present. The DME allows for a wide potential range and surface regeneration between drops.Polarography



PolarographyMadhurishelar239
Ěý
This document discusses polarography, which is a technique for analyzing solutions using two electrodes - a dropping mercury working electrode and a reference electrode. It provides details on:
1. How polarography works by applying a voltage to induce a redox reaction and measuring the resulting current.
2. The components needed, including the dropping mercury electrode, reference electrode, and a supporting electrolyte.
3. How polarograms are generated by plotting current vs. applied voltage and the different regions that can be seen on a polarogram.
4. Factors that influence the diffusion current measured, such as concentration of the analyte, diffusion coefficient, and drop lifetime. Equations for calculating diffusion current are also presented.Error in chemical analysis



Error in chemical analysisSuresh Selvaraj
Ěý
Mean, Median, Accuracy, Precision, Absolute error, Relative error, Significant Figures, Normal error curveDiazotization titrations



Diazotization titrationsrdeepthi1
Ěý
The document provides information about diazotization titrations. It discusses the principle, theory, procedure, end point detection, factors affecting, applications, and advantages/disadvantages of diazotization titrations. The key points are:
- Diazotization titrations involve the reaction of a primary aromatic amine with sodium nitrite in acidic medium to form a diazonium salt, which is then titrated.
- The end point is detected using an external indicator like starch iodide paper or electrochemically.
- Factors like acid concentration, temperature, and reaction time must be controlled.
- It can be used to determine drugs and compounds containingPolarography- Pharmaceutical Analysis



Polarography- Pharmaceutical AnalysisSanchit Dhankhar
Ěý
The earliest voltammetric technique
Heyrovsky invented the original polarographic method in 1922, conventional direct current polarography (DCP).
It employs a dropping mercury electrode (DME) to continuously renew the electrode surface.
Diffusion is the mechanism of mass transport.
When an external potential is applied to a cell
containing a reducing substance such as CdCl2,
The following reaction will occur:
Cd2+ + 2e + Hg = Cd(Hg)
The technique depends on increasing the applied
voltage at a steady rate and simultaneously
record photographically the current-voltage
curve (polarogram)
The apparatus used is called a polarograph .
When an external potential is applied to a cell
containing a reducing substance such as CdCl2,
The following reaction will occur:
Cd2+ + 2e + Hg = Cd(Hg)
The technique depends on increasing the applied
voltage at a steady rate and simultaneously
record photographically the current-voltage
curve (polarogram)
The apparatus used is called a polarograph .
Capillary tube about 10-15cm
Int. diameter of 0.05mm
A vertical distance being maintained betwwen DME and the solution
Drop time of 1-5 seconds
Drop diameter 0.5mm
The supporting electrolyte
is a solution of (KNO3, NaCl, Na3PO4) in which the sample (which must be electroactive) is dissolved.
Function of the supporting electrolyte
It raises the conductivity of the solution.
It carries the bulk of the current so prevent the
migration of electroactive materials to working
electrode.
It may control pH
It may associate with the electroactive solute as
in the complexing of the metal ions by ligands.
PA-I Potentiometry. (HRB)



PA-I Potentiometry. (HRB)Harshadaa bafna
Ěý
Potentiometry involves measuring the potential or electromotive force of a sample solution using an electrochemical cell containing a reference electrode and an indicator electrode. The potential is directly proportional to the ion concentration in the solution. Common reference electrodes include the standard hydrogen electrode, silver chloride electrode, and saturated calomel electrode. Indicator electrodes can be metal electrodes or ion-selective electrodes like the glass membrane pH electrode. Potentiometric titration determines the concentration of an analyte by measuring the potential change as a titrant is added, with the endpoint indicated by an abrupt potential shift. Applications of potentiometry include acid-base, redox, complexometric, and precipitation titrations.Conductometry



ConductometryISF COLLEGE OF PHARMACY MOGA
Ěý
It is an electrochemical method of analysis used for the determination or measurement of the electrical conductance of an electrolyte solution by means of a conductometer.
Electric conductivity of an electrolyte solution depends on :
Type of ions (cations, anions, singly or doubly charged
Concentration of ions
Temperature
Mobility of ions
The main principle involved in this method is that the movement of the ions creates the electrical conductivity. The movement of the ions is mainly depended on the concentration of the ions.
The electric conductance in accordance with ohms law which states that the strength of current (i) passing through conductor is directly proportional to potential difference & inversely to resistance.
i =V/R
Conductometry



ConductometryMadhu Lika
Ěý
Conductometric analysis measures the electrical conductivity of solutions to determine analyte concentration. It works by measuring how easily ions move through the solution when a current is applied. There are several types of conductometric titrations including acid-base, redox, and complexometric titrations. Conductometric titrations can determine the endpoint graphically without needing indicators and work well for colored, weak, or turbid solutions. The conductivity is measured using a conductometer with conductivity cells and platinum electrodes to apply a current and measure the solution's resistance.Polarography



PolarographySPCGC AJMER
Ěý
Polarography is an electroanalytical technique invented in 1922 by Jaroslav Heyrovsky for which he won the Nobel Prize. It involves measuring the current in a solution under an applied potential using a dropping mercury electrode and a reference electrode such as SCE. Mercury is used as the working electrode due to its wide negative potential range and ability to regenerate its surface. A polarogram is generated by plotting current versus applied potential, showing residual, diffusion, and limiting currents. Polarography can be used for qualitative and quantitative analysis of metals, drugs, and other compounds.AMPEROMETRY



AMPEROMETRYAkshayAkotkar
Ěý
Amperometric titration involves measuring the electric current produced by a titration reaction while keeping the voltage constant between electrodes. It can determine the endpoint of titrations involving an electroreducible ion being titrated with a counter ion. The diffusion current is measured and plotted against the titrant volume added. At the endpoint, there is a sharp change in current. Amperometric titration offers advantages like rapid analysis, ability to work with dilute solutions, and determination of insoluble substances. It finds applications in areas like determining water content and quantification of ions.AMPEROMETRY and AMPEROMETRIC TITRATIONS



AMPEROMETRY and AMPEROMETRIC TITRATIONSEinstein kannan
Ěý
It contains what is amperometry and where it will be derived and what is the principle behind the amperometry. Instrumentation of amperometry and the purpose of dipping mercury electrode and rotating platinum electrode. The advantage over rotating platinum electrodes. Amperometric titration curves for reducible ions and non-reducible ions. What tells the Ilkovic equation and how it relates to the amperometry is also included. Applications, advantages, and disadvantages of amperometric titration are also included. Questions related to amperometry and amperometric titration are given for practice. The contents taken from the websites are also given.PA-I Complexometric titration.(HRB)



PA-I Complexometric titration.(HRB)Harshadaa bafna
Ěý
Complexometric titration involves the titration of a metal ion solution with a chelating agent or ligand until the metal ion forms a stable complex. It is useful for determining mixtures of metal ions. The document discusses various types of complexometric titrations including direct titration, back titration, and replacement titration. It also covers the use of metal ion indicators, masking and demasking reagents, and provides examples of complexometric titration for determining compounds like magnesium sulfate, calcium gluconate, and auric ions in ores.Polarography



PolarographyISF COLLEGE OF PHARMACY MOGA
Ěý
Polarographic technique is applied for the qualitative or quantitative analysis of electroreducible or oxidisable elements or groups.
It is an electromechanical technique of analyzing solutions that measures the current flowing between two electrodes in the solution as well as the gradually increasing applied voltage to determine respectively the concentration of a solute and its nature.
The principle in polarography is that a gradually increasing negative potential (voltage) is applied between a polarisable and non-polarisable electrode and the corresponding current is recorded.
Polarisable electrode: Dropping Mercury electrode
Non-polarisable electrode: Saturated Calomel electrode
From the current-voltage curve (Sigmoid shape), qualitative and quantitative analysis can be performed. This technique is called as polarography, the instrument used is called as polarograph and the current-voltage curve recorded is called as polarogramPotentiometry ppt



Potentiometry pptPoornima Santhosh
Ěý
it is about the description of Potentiometry, EMF, Types of Electrodes, Measurement of Electrode potential and applications of Potentiometry.Voltammetry and Polarography



Voltammetry and PolarographyMelakuMetto
Ěý
Voltammetry is a technique where a time-dependent potential is applied to an electrochemical cell and the current is measured as a function of the applied potential. This results in a voltammogram which provides qualitative and quantitative information about redox reactions. The earliest technique was polarography developed in the 1920s. Modern voltammetry uses a three-electrode system with various excitation signals applied. Common techniques include normal pulse polarography, differential pulse polarography, staircase polarography and square wave polarography which have better sensitivity than normal polarography. The shape of the voltammetric wave depends on factors like the reversibility of the redox reaction. The diffusion current occurs at very negative potentials where the reaction rate is controlled by diffusionPotentiometry, Electrochemical cell, construction and working of indicator an...



Potentiometry, Electrochemical cell, construction and working of indicator an...Vandana Devesh Sharma
Ěý
Potentiometry - Electrochemical cell -Construction and working of reference (Standard hydrogen, silver chloride electrode and calomel electrode)
Indicator electrodes (metal electrodes and glass electrode)
Methods to determine end point of potentiometric titration
and applications
Potentiometry isĚýthe method to find the concentration of solute in
A given solution by measuring the potential between two Electrodes
(reference and Indicator electrode) . Potentiometric titration involves
the measurement of the potential of the indicator electrode and
reference electrode.
In potentiometric titration reference and indicator electrodes are
immersed in the solution of particular analyte (titrand) and
potential of indicator electrode is measured with relation to
reference electrode.
Titrant is added in analyte (Titrand) and change in potential is noted
down.
At the end point there is sharp change in potential on indicator
electrode.
Graph is plotted between the indicator electrode potential and
volume of titrant added.
This method is used for determination of sharp end point.
Types of Potentiometric Titration
1. Acid-base titration 2. Redox TitrationĚý 3.Complexometric titration 4. Precipitation Titration
polarography



polarographyfathimashahul22
Ěý
Polarography uses a dropping mercury electrode (DME) to measure the current flowing through an electrochemical cell as a function of the applied potential. A polarogram plots this current versus potential and provides qualitative and quantitative information about species undergoing oxidation or reduction reactions. Jaroslav Heyrovsky invented the polarographic method in 1922 and won the Nobel Prize for his contributions to electroanalytical chemistry. All modern voltammetric methods originate from polarography. The DME provides advantages like a reproducible surface area and the ability to form amalgams with metal ions.Acid base titration



Acid base titrationmeraj khan
Ěý
This document discusses acid-base theories and titration. It covers:
1) Arrhenius, Bronsted-Lowry, and Lewis acid-base theories.
2) Types of acids and bases as strong or weak.
3) The law of mass action and dissociation constants.
4) Neutralization curves for different types of acid-base titrations and the pH at equivalence points.
5) Choice of indicators for different titrations and mixed indicators.Gravimetric Analysis.pptx



Gravimetric Analysis.pptxSajidHussain495712
Ěý
This document discusses gravimetric analysis methods. It defines gravimetric analysis as isolating and weighing an element or compound in pure form to determine the quantity present. The main types discussed are precipitation gravimetry, electrogravimetry, and volatilization gravimetry. Precipitation gravimetry, the formation of an insoluble precipitate, is explained in detail including factors that influence successful precipitation and purity of the precipitate. Advantages include high precision and accuracy, while disadvantages include being time-consuming and requiring clean glassware and accurate weighing. An example of barium chloride estimation by precipitating and weighing barium sulfate is also provided.Potentiometry titration



Potentiometry titrationAnoop Singh
Ěý
This document provides information on potentiometry and potentiometric titration. It discusses the basic principles of potentiometry including electrode potentials and how a potential difference is established between an electrode and solution. It describes the instrumentation used including reference electrodes like calomel and silver-silver chloride electrodes and indicator electrodes like metal, glass membrane, and quinhydrone electrodes. It also discusses different types of potentiometric titrations and provides examples of applications for potentiometry in various industries.Electroanalytical Methods of analysis



Electroanalytical Methods of analysisShanta Majumder
Ěý
The document provides information about electroanalytical methods of analysis. It defines electroanalytical methods as techniques that study analytes by measuring potentials or currents in an electrochemical cell containing the analyte. It discusses various types of electroanalytical techniques including potentiometry, voltammetry, and Karl Fischer titration. It provides details on the principles, instrumentation, applications, and advantages of these analytical methods.Estimate the amount Ni by EDTA



Estimate the amount Ni by EDTAMithil Fal Desai
Ěý
This document provides instructions for estimating the amount of nickel (Ni) in a sample using complexometric titration with EDTA. The procedure involves:
1) Standardizing a solution of EDTA against zinc sulfate to determine its concentration.
2) Dissolving a nickel sample and filtering the solution.
3) Titrating an aliquot of the nickel solution against EDTA using murexide indicator, which changes color from yellow to red at the endpoint.
4) Calculating the concentration of nickel in the original sample and percentage of nickel in the complex from titration results.Potentiometry



PotentiometryKaranvir Rajput
Ěý
Potentiometry, working, electrode, ion selective electrods, modern pharmaceutical analytical techniquesNephelometry and turbidimetry



Nephelometry and turbidimetryushaSanmugaraj
Ěý
it consists of material for nephelometry and turbidimetry introduction, principle, instrumentation and applications.Amperometry



AmperometryArpitSuralkar
Ěý
This document discusses amperometric titration, which is an electrochemical titration method that measures current under a constant applied voltage. It explains the principle that the current passing through an indicator electrode is measured during titration as the concentration of electroreducible ions changes. The document outlines the conditions, apparatus used including dropping mercury and rotating platinum microelectrodes, types of amperometric titrations, advantages such as ability to analyze reducible and non-reducible ions, applications including HPLC detection, and disadvantages like inaccurate results from foreign substances.PA-I Condutometry.(HRB)



PA-I Condutometry.(HRB)Harshadaa bafna
Ěý
This document provides an overview of conductometry. It discusses how conductometry measures the conductance of electrolyte solutions using a conductivity cell and conductometer. It describes different types of conductivity cells and how conductometric titrations work by measuring changes in conductance during titrations. Examples of various acid-base titrations are given. Conductometric titrations can be used to analyze many different samples and have advantages like not requiring indicators. Applications include measuring water pollution, food analyses, and more.Potentiometry



PotentiometryGagan Deep
Ěý
Potentiometry is an analytical technique that measures the potential of electrochemical cells without drawing current. It involves using a reference electrode with a known potential and an indicator electrode whose potential varies with analyte concentration. The cell potential is measured and related to concentration using the Nernst equation. Common reference electrodes include the standard hydrogen electrode and saturated calomel electrode. Glass membrane and ion-selective electrodes are often used as indicator electrodes to detect specific ions like hydrogen or fluoride ions. Potentiometry finds applications in clinical analysis, environmental monitoring, and titration experiments.Potentiometry



Potentiometryravishankar05
Ěý
Potentiometry uses a reference electrode and an indicator electrode to measure the potential difference in a sample solution. When the electrodes are placed in the solution, the potential is generated based on the concentration of ions present. There are several types of potentiometric titrations including acid-base, redox, complexometric, and precipitation titrations. Potentiometry has many applications in fields like clinical chemistry, environmental analysis, potentiometric titrations, agriculture, detergent manufacturing, food processing and more. It is used to analyze important ions and determine equivalence points during titrations.More Related Content
What's hot (20)
Conductometry



ConductometryISF COLLEGE OF PHARMACY MOGA
Ěý
It is an electrochemical method of analysis used for the determination or measurement of the electrical conductance of an electrolyte solution by means of a conductometer.
Electric conductivity of an electrolyte solution depends on :
Type of ions (cations, anions, singly or doubly charged
Concentration of ions
Temperature
Mobility of ions
The main principle involved in this method is that the movement of the ions creates the electrical conductivity. The movement of the ions is mainly depended on the concentration of the ions.
The electric conductance in accordance with ohms law which states that the strength of current (i) passing through conductor is directly proportional to potential difference & inversely to resistance.
i =V/R
Conductometry



ConductometryMadhu Lika
Ěý
Conductometric analysis measures the electrical conductivity of solutions to determine analyte concentration. It works by measuring how easily ions move through the solution when a current is applied. There are several types of conductometric titrations including acid-base, redox, and complexometric titrations. Conductometric titrations can determine the endpoint graphically without needing indicators and work well for colored, weak, or turbid solutions. The conductivity is measured using a conductometer with conductivity cells and platinum electrodes to apply a current and measure the solution's resistance.Polarography



PolarographySPCGC AJMER
Ěý
Polarography is an electroanalytical technique invented in 1922 by Jaroslav Heyrovsky for which he won the Nobel Prize. It involves measuring the current in a solution under an applied potential using a dropping mercury electrode and a reference electrode such as SCE. Mercury is used as the working electrode due to its wide negative potential range and ability to regenerate its surface. A polarogram is generated by plotting current versus applied potential, showing residual, diffusion, and limiting currents. Polarography can be used for qualitative and quantitative analysis of metals, drugs, and other compounds.AMPEROMETRY



AMPEROMETRYAkshayAkotkar
Ěý
Amperometric titration involves measuring the electric current produced by a titration reaction while keeping the voltage constant between electrodes. It can determine the endpoint of titrations involving an electroreducible ion being titrated with a counter ion. The diffusion current is measured and plotted against the titrant volume added. At the endpoint, there is a sharp change in current. Amperometric titration offers advantages like rapid analysis, ability to work with dilute solutions, and determination of insoluble substances. It finds applications in areas like determining water content and quantification of ions.AMPEROMETRY and AMPEROMETRIC TITRATIONS



AMPEROMETRY and AMPEROMETRIC TITRATIONSEinstein kannan
Ěý
It contains what is amperometry and where it will be derived and what is the principle behind the amperometry. Instrumentation of amperometry and the purpose of dipping mercury electrode and rotating platinum electrode. The advantage over rotating platinum electrodes. Amperometric titration curves for reducible ions and non-reducible ions. What tells the Ilkovic equation and how it relates to the amperometry is also included. Applications, advantages, and disadvantages of amperometric titration are also included. Questions related to amperometry and amperometric titration are given for practice. The contents taken from the websites are also given.PA-I Complexometric titration.(HRB)



PA-I Complexometric titration.(HRB)Harshadaa bafna
Ěý
Complexometric titration involves the titration of a metal ion solution with a chelating agent or ligand until the metal ion forms a stable complex. It is useful for determining mixtures of metal ions. The document discusses various types of complexometric titrations including direct titration, back titration, and replacement titration. It also covers the use of metal ion indicators, masking and demasking reagents, and provides examples of complexometric titration for determining compounds like magnesium sulfate, calcium gluconate, and auric ions in ores.Polarography



PolarographyISF COLLEGE OF PHARMACY MOGA
Ěý
Polarographic technique is applied for the qualitative or quantitative analysis of electroreducible or oxidisable elements or groups.
It is an electromechanical technique of analyzing solutions that measures the current flowing between two electrodes in the solution as well as the gradually increasing applied voltage to determine respectively the concentration of a solute and its nature.
The principle in polarography is that a gradually increasing negative potential (voltage) is applied between a polarisable and non-polarisable electrode and the corresponding current is recorded.
Polarisable electrode: Dropping Mercury electrode
Non-polarisable electrode: Saturated Calomel electrode
From the current-voltage curve (Sigmoid shape), qualitative and quantitative analysis can be performed. This technique is called as polarography, the instrument used is called as polarograph and the current-voltage curve recorded is called as polarogramPotentiometry ppt



Potentiometry pptPoornima Santhosh
Ěý
it is about the description of Potentiometry, EMF, Types of Electrodes, Measurement of Electrode potential and applications of Potentiometry.Voltammetry and Polarography



Voltammetry and PolarographyMelakuMetto
Ěý
Voltammetry is a technique where a time-dependent potential is applied to an electrochemical cell and the current is measured as a function of the applied potential. This results in a voltammogram which provides qualitative and quantitative information about redox reactions. The earliest technique was polarography developed in the 1920s. Modern voltammetry uses a three-electrode system with various excitation signals applied. Common techniques include normal pulse polarography, differential pulse polarography, staircase polarography and square wave polarography which have better sensitivity than normal polarography. The shape of the voltammetric wave depends on factors like the reversibility of the redox reaction. The diffusion current occurs at very negative potentials where the reaction rate is controlled by diffusionPotentiometry, Electrochemical cell, construction and working of indicator an...



Potentiometry, Electrochemical cell, construction and working of indicator an...Vandana Devesh Sharma
Ěý
Potentiometry - Electrochemical cell -Construction and working of reference (Standard hydrogen, silver chloride electrode and calomel electrode)
Indicator electrodes (metal electrodes and glass electrode)
Methods to determine end point of potentiometric titration
and applications
Potentiometry isĚýthe method to find the concentration of solute in
A given solution by measuring the potential between two Electrodes
(reference and Indicator electrode) . Potentiometric titration involves
the measurement of the potential of the indicator electrode and
reference electrode.
In potentiometric titration reference and indicator electrodes are
immersed in the solution of particular analyte (titrand) and
potential of indicator electrode is measured with relation to
reference electrode.
Titrant is added in analyte (Titrand) and change in potential is noted
down.
At the end point there is sharp change in potential on indicator
electrode.
Graph is plotted between the indicator electrode potential and
volume of titrant added.
This method is used for determination of sharp end point.
Types of Potentiometric Titration
1. Acid-base titration 2. Redox TitrationĚý 3.Complexometric titration 4. Precipitation Titration
polarography



polarographyfathimashahul22
Ěý
Polarography uses a dropping mercury electrode (DME) to measure the current flowing through an electrochemical cell as a function of the applied potential. A polarogram plots this current versus potential and provides qualitative and quantitative information about species undergoing oxidation or reduction reactions. Jaroslav Heyrovsky invented the polarographic method in 1922 and won the Nobel Prize for his contributions to electroanalytical chemistry. All modern voltammetric methods originate from polarography. The DME provides advantages like a reproducible surface area and the ability to form amalgams with metal ions.Acid base titration



Acid base titrationmeraj khan
Ěý
This document discusses acid-base theories and titration. It covers:
1) Arrhenius, Bronsted-Lowry, and Lewis acid-base theories.
2) Types of acids and bases as strong or weak.
3) The law of mass action and dissociation constants.
4) Neutralization curves for different types of acid-base titrations and the pH at equivalence points.
5) Choice of indicators for different titrations and mixed indicators.Gravimetric Analysis.pptx



Gravimetric Analysis.pptxSajidHussain495712
Ěý
This document discusses gravimetric analysis methods. It defines gravimetric analysis as isolating and weighing an element or compound in pure form to determine the quantity present. The main types discussed are precipitation gravimetry, electrogravimetry, and volatilization gravimetry. Precipitation gravimetry, the formation of an insoluble precipitate, is explained in detail including factors that influence successful precipitation and purity of the precipitate. Advantages include high precision and accuracy, while disadvantages include being time-consuming and requiring clean glassware and accurate weighing. An example of barium chloride estimation by precipitating and weighing barium sulfate is also provided.Potentiometry titration



Potentiometry titrationAnoop Singh
Ěý
This document provides information on potentiometry and potentiometric titration. It discusses the basic principles of potentiometry including electrode potentials and how a potential difference is established between an electrode and solution. It describes the instrumentation used including reference electrodes like calomel and silver-silver chloride electrodes and indicator electrodes like metal, glass membrane, and quinhydrone electrodes. It also discusses different types of potentiometric titrations and provides examples of applications for potentiometry in various industries.Electroanalytical Methods of analysis



Electroanalytical Methods of analysisShanta Majumder
Ěý
The document provides information about electroanalytical methods of analysis. It defines electroanalytical methods as techniques that study analytes by measuring potentials or currents in an electrochemical cell containing the analyte. It discusses various types of electroanalytical techniques including potentiometry, voltammetry, and Karl Fischer titration. It provides details on the principles, instrumentation, applications, and advantages of these analytical methods.Estimate the amount Ni by EDTA



Estimate the amount Ni by EDTAMithil Fal Desai
Ěý
This document provides instructions for estimating the amount of nickel (Ni) in a sample using complexometric titration with EDTA. The procedure involves:
1) Standardizing a solution of EDTA against zinc sulfate to determine its concentration.
2) Dissolving a nickel sample and filtering the solution.
3) Titrating an aliquot of the nickel solution against EDTA using murexide indicator, which changes color from yellow to red at the endpoint.
4) Calculating the concentration of nickel in the original sample and percentage of nickel in the complex from titration results.Potentiometry



PotentiometryKaranvir Rajput
Ěý
Potentiometry, working, electrode, ion selective electrods, modern pharmaceutical analytical techniquesNephelometry and turbidimetry



Nephelometry and turbidimetryushaSanmugaraj
Ěý
it consists of material for nephelometry and turbidimetry introduction, principle, instrumentation and applications.Amperometry



AmperometryArpitSuralkar
Ěý
This document discusses amperometric titration, which is an electrochemical titration method that measures current under a constant applied voltage. It explains the principle that the current passing through an indicator electrode is measured during titration as the concentration of electroreducible ions changes. The document outlines the conditions, apparatus used including dropping mercury and rotating platinum microelectrodes, types of amperometric titrations, advantages such as ability to analyze reducible and non-reducible ions, applications including HPLC detection, and disadvantages like inaccurate results from foreign substances.PA-I Condutometry.(HRB)



PA-I Condutometry.(HRB)Harshadaa bafna
Ěý
This document provides an overview of conductometry. It discusses how conductometry measures the conductance of electrolyte solutions using a conductivity cell and conductometer. It describes different types of conductivity cells and how conductometric titrations work by measuring changes in conductance during titrations. Examples of various acid-base titrations are given. Conductometric titrations can be used to analyze many different samples and have advantages like not requiring indicators. Applications include measuring water pollution, food analyses, and more.Potentiometry, Electrochemical cell, construction and working of indicator an...



Potentiometry, Electrochemical cell, construction and working of indicator an...Vandana Devesh Sharma
Ěý
Similar to potentiometric titration ^.pptx (20)
Potentiometry



PotentiometryGagan Deep
Ěý
Potentiometry is an analytical technique that measures the potential of electrochemical cells without drawing current. It involves using a reference electrode with a known potential and an indicator electrode whose potential varies with analyte concentration. The cell potential is measured and related to concentration using the Nernst equation. Common reference electrodes include the standard hydrogen electrode and saturated calomel electrode. Glass membrane and ion-selective electrodes are often used as indicator electrodes to detect specific ions like hydrogen or fluoride ions. Potentiometry finds applications in clinical analysis, environmental monitoring, and titration experiments.Potentiometry



Potentiometryravishankar05
Ěý
Potentiometry uses a reference electrode and an indicator electrode to measure the potential difference in a sample solution. When the electrodes are placed in the solution, the potential is generated based on the concentration of ions present. There are several types of potentiometric titrations including acid-base, redox, complexometric, and precipitation titrations. Potentiometry has many applications in fields like clinical chemistry, environmental analysis, potentiometric titrations, agriculture, detergent manufacturing, food processing and more. It is used to analyze important ions and determine equivalence points during titrations.Potentiometry



PotentiometryZainab&Sons
Ěý
Potentiometry involves measuring electrode potentials using a reference electrode and indicator electrode. The reference electrode maintains a constant potential while the indicator electrode's potential varies with analyte concentration. Common reference electrodes include the saturated calomel electrode and silver-silver chloride electrode. Indicator electrodes include pH electrodes, ion-selective electrodes, and redox electrodes. Potentiometric measurements are used in clinical chemistry, environmental monitoring, titrations, and various industrial applications like food processing.POTENTIOMTRY.ppt



POTENTIOMTRY.pptMeenakshi Dhanawat
Ěý
Potentiometric titration is a chemical analysis technique that relies on measuring the electromotive force (EMF) of a solution using indicator and reference electrodes. The EMF depends on the ions present in the solution. During titration, the EMF is measured after each addition of titrant and graphed versus volume added. There are four main types of potentiometric titration: acid-base titration determines concentration by neutralization, redox titration involves a redox reaction, complexometric titration forms colored complexes, and precipitation titration forms an insoluble precipitate.Potentiometry.pptx



Potentiometry.pptxYunesalsayadi
Ěý
Potentiometry: Electrical potential, electrochemical cell, reference electrodes, indicator
electrodes, measurement of potential and Ph, construction and working of electrodes,
Potentiometric titrations, methods of detecting end point, Karl Fischer titration. Potentiometry.pptx



Potentiometry.pptxAida Docena
Ěý
This document discusses potentiometry, which uses the potential between two electrodes to determine the concentration of a solute. It describes the theory behind potentiometric measurements involving charge separation at interfaces. There are two main types of indicator electrodes: metallic direct indicator electrodes whose response involves redox reactions, and membrane electrodes or ion-selective electrodes. The document outlines the construction, working principles, and applications of potentiometry including characteristics, potentiometric cells, and reference electrodes.electrochemical methods of analysis of complex samples



electrochemical methods of analysis of complex samplesKipkiruiKen
Ěý
methods of electrochemical anlysis of sampleIon Selective Electrode



Ion Selective ElectrodeWritu Bashyal
Ěý
This document provides information on ion selective electrodes (ISEs). It discusses the history of ISE development from early pH electrodes to modern commercial applications. The basic components and functioning of ISEs are described, including the electrochemical cell setup with an ion selective membrane and reference electrode. Different types of membranes - glass, crystal, gas-sensing, and polymer - are outlined. Examples are given of specific ions that can be measured with different ISE configurations. The document concludes by discussing the mechanisms of ion exchange and selectivity in polymer membrane electrodes.potentiometry & ion selective electode



potentiometry & ion selective electodersgokani
Ěý
This document discusses potentiometry and ion selective electrodes. It begins by explaining that potentiometry measures the potential of an electrochemical cell under static conditions without drawing current. An ion selective electrode uses a selective membrane to measure the concentration of specific ions based on the potential difference between an indicator and reference electrode. The document then describes different types of reference electrodes, indicator electrodes, and ion selective electrodes like glass membrane, solid state, liquid membrane and gas sensing electrodes. It concludes by discussing applications in clinical chemistry, environmental analysis and food processing and advantages like speed and low cost and limitations like precision and interference issues.Potentiometry



PotentiometrySELINA SRAVANTHI
Ěý
Potentiometry is an electrochemical method used to measure the electrical potential of an electrolyte solution. It is based on the Nernst equation, which relates the potential of an electrochemical cell to the concentration of ions. A potentiometric cell consists of a reference electrode with a fixed potential and an indicator electrode that responds to the analyte. The potential difference between the electrodes is measured and can be used to determine the concentration of the analyte. Common reference electrodes include the standard hydrogen electrode, saturated calomel electrode, and silver/silver chloride electrode.Potentiometry ppt By Chand.pptx



Potentiometry ppt By Chand.pptxJiwaji University, Gwalior
Ěý
(All about potentiometry) Potentiometry, Reference Electrode, Indicator Electrode, Ion Selective Electrode, Potentiometric titration, Application, Advantages, Disadvantages etc.Basics of Electrochemistry and Electrochemical Measurements



Basics of Electrochemistry and Electrochemical MeasurementsHalavath Ramesh
Ěý
A potentiostat is an electronic instrument that controls the voltage difference between a working electrode and a reference electrode by injecting current through an auxiliary electrode. It is used to apply a potential to an electrochemical cell and measure the resulting current. A potentiostat requires a three-electrode cell with a working electrode, reference electrode, and counter electrode. The working electrode is where the potential is controlled and current is measured. Common reference electrodes include the saturated calomel electrode and silver/silver chloride electrode, which maintain a constant potential. The counter electrode completes the circuit by allowing current to flow out of the cell. Potentiostats are used to study electrochemical reactions and processes.Electrochemical methods: Environmental Analysis 



Electrochemical methods: Environmental Analysis Almas Tamake
Ěý
Electrochemical methods are analytical techniques that use measurements of potential, charge, or current to determine an analyte's concentration or characterize its reactivity. They are divided into five major groups: potentiometry, voltammetry, coulometry, conductometry, and dielectrometry. Potentiometry measures the potential of a solution between two electrodes to relate it to an analyte's concentration. Voltammetry applies a constant or varying potential to measure the resulting current using a three-electrode system. Coulometry measures material deposited on an electrode during an electrochemical reaction using Faraday's laws. Conductometry measures the electrical conductivity of electrolyte solutions. Electrochemical techniques can be used to obtain thermodynamic data, study unstableBasics of Voltammetry and Potentiometry 



Basics of Voltammetry and Potentiometry Pranay Krishnan
Ěý
This document provides an overview of voltammetry and potentiometry techniques. It discusses the history and development of voltammetry, which involves measuring current as a function of applied potential. Common types of voltammetry include linear sweep, cyclic, and stripping voltammetry. The document also describes the basic components of a voltammetry system, including the working, reference, and counter electrodes. Finally, it provides a brief introduction to potentiometry and its applications in titration and measuring concentration, activity, and pH.POTENTIOMETRY.pdf



POTENTIOMETRY.pdfSaqibShaik2
Ěý
This document provides an overview of electrochemistry and electrochemical cells. It defines electrochemistry as the study of the relationship between chemical transformations and electrical energy. It describes the two main types of electrochemical cells - electrolytic cells, which convert electrical to chemical energy, and galvanic/voltaic cells, which convert chemical to electrical energy. Key aspects of electrochemical cells covered include the electrodes, electrode charges, redox reactions, cell notation, salt bridges, cell potential, and reference electrodes. The document also discusses indicator electrodes, such as glass pH electrodes and potentiometric titration methods.Electrochemical method of analysis



Electrochemical method of analysisSiham Abdallaha
Ěý
Electrochemical methods are analytical techniques that use measurements of potential, charge, or current to determine an analyte's concentration or characterize its reactivity. There are several types of electrochemical methods including potentiometry, voltammetry, coulometry, conductometry, and dielectrometry. Potentiometry measures the potential of a solution between two electrodes and relates the potential to analyte concentrations. Voltammetry applies a constant or varying potential at an electrode and measures the resulting current. Coulometry completely converts an analyte from one oxidation state to another by applying current or potential and measuring the total current passed. Potentiometric titration uses two electrodes to measure the potential across a solution during a titration rather than usingpotentiometry



potentiometryNorasheila Mohd Saad
Ěý
This document discusses the principles of potentiometric measurement. Potentiometry involves measuring the potential of an electrochemical cell under conditions of zero current flow, allowing the cell composition to remain unchanged. The potential is related to analyte concentration by the Nernst equation. Potentiometric cells consist of a sensing electrode and a reference electrode separated by a salt bridge. The potential difference between the electrodes corresponds to analyte levels. Common sensing electrodes include ion-selective electrodes and metallic electrodes like silver or copper that respond to specific ions.Recently uploaded (20)
Year 10 The Senior Phase Session 3 Term 1.pptx



Year 10 The Senior Phase Session 3 Term 1.pptxmansk2
Ěý
Year 10 The Senior Phase Session 3 Term 1.pptxN.C. DPI's 2023 Language Diversity Briefing



N.C. DPI's 2023 Language Diversity BriefingMebane Rash
Ěý
The number of languages spoken in NC public schools.QuickBooks Desktop to QuickBooks Online How to Make the Move



QuickBooks Desktop to QuickBooks Online How to Make the MoveTechSoup
Ěý
If you use QuickBooks Desktop and are stressing about moving to QuickBooks Online, in this webinar, get your questions answered and learn tips and tricks to make the process easier for you.
Key Questions:
* When is the best time to make the shift to QuickBooks Online?
* Will my current version of QuickBooks Desktop stop working?
* I have a really old version of QuickBooks. What should I do?
* I run my payroll in QuickBooks Desktop now. How is that affected?
*Does it bring over all my historical data? Are there things that don't come over?
* What are the main differences between QuickBooks Desktop and QuickBooks Online?
* And moreChapter 3. Social Responsibility and Ethics in Strategic Management.pptx



Chapter 3. Social Responsibility and Ethics in Strategic Management.pptxRommel Regala
Ěý
This course provides students with a comprehensive understanding of strategic management principles, frameworks, and applications in business. It explores strategic planning, environmental analysis, corporate governance, business ethics, and sustainability. The course integrates Sustainable Development Goals (SDGs) to enhance global and ethical perspectives in decision-making.How to Modify Existing Web Pages in Odoo 18



How to Modify Existing Web Pages in Odoo 18Celine George
Ěý
In this slide, we’ll discuss on how to modify existing web pages in Odoo 18. Web pages in Odoo 18 can also gather user data through user-friendly forms, encourage interaction through engaging features. How to use Init Hooks in Odoo 18 - Odoo şÝşÝߣs



How to use Init Hooks in Odoo 18 - Odoo şÝşÝߣsCeline George
Ěý
In this slide, we’ll discuss on how to use Init Hooks in Odoo 18. In Odoo, Init Hooks are essential functions specified as strings in the __init__ file of a module.SOCIAL CHANGE(a change in the institutional and normative structure of societ...



SOCIAL CHANGE(a change in the institutional and normative structure of societ...DrNidhiAgarwal
Ěý
This PPT is showing the effect of social changes in human life and it is very understandable to the students with easy language.in this contents are Itroduction, definition,Factors affecting social changes ,Main technological factors, Social change and stress , what is eustress and how social changes give impact of the human's life.Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Finals of Rass MELAI : a Music, Entertainment, Literature, Arts and Internet Culture Quiz organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. Useful environment methods in Odoo 18 - Odoo şÝşÝߣs



Useful environment methods in Odoo 18 - Odoo şÝşÝߣsCeline George
Ěý
In this slide we’ll discuss on the useful environment methods in Odoo 18. In Odoo 18, environment methods play a crucial role in simplifying model interactions and enhancing data processing within the ORM framework.Adventure Activities Final By H R Gohil Sir



Adventure Activities Final By H R Gohil SirGUJARATCOMMERCECOLLE
Ěý
Adventure Activities Final By H R Gohil SirHow to Setup WhatsApp in Odoo 17 - Odoo şÝşÝߣs



How to Setup WhatsApp in Odoo 17 - Odoo şÝşÝߣsCeline George
Ěý
Integrate WhatsApp into Odoo using the WhatsApp Business API or third-party modules to enhance communication. This integration enables automated messaging and customer interaction management within Odoo 17.Kaun TALHA quiz Prelims - El Dorado 2025



Kaun TALHA quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Prelims of Kaun TALHA : a Travel, Architecture, Lifestyle, Heritage and Activism quiz, organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. Research & Research Methods: Basic Concepts and Types.pptx



Research & Research Methods: Basic Concepts and Types.pptxDr. Sarita Anand
Ěý
This ppt has been made for the students pursuing PG in social science and humanities like M.Ed., M.A. (Education), Ph.D. Scholars. It will be also beneficial for the teachers and other faculty members interested in research and teaching research concepts.Kaun TALHA quiz Finals -- El Dorado 2025



Kaun TALHA quiz Finals -- El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Finals of Kaun TALHA : a Travel, Architecture, Lifestyle, Heritage and Activism quiz, organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. Digital Tools with AI for e-Content Development.pptx



Digital Tools with AI for e-Content Development.pptxDr. Sarita Anand
Ěý
This ppt is useful for not only for B.Ed., M.Ed., M.A. (Education) or any other PG level students or Ph.D. scholars but also for the school, college and university teachers who are interested to prepare an e-content with AI for their students and others.Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Kaun TALHA quiz Prelims - El Dorado 2025



Kaun TALHA quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Kaun TALHA quiz Finals -- El Dorado 2025



Kaun TALHA quiz Finals -- El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
potentiometric titration ^.pptx
- 1. Potentiometry, principle and Working Nitin pandey M.pharm 1st year Pharmaceutical chemistry
- 2. Introduction • Potentiometry is the method to find the concentration of solute in a given solution by measuring the potential between two electrodes. • As the name suggests, potentiometric titration involves the measurement of the potential of the indicator electrode and reference electrode. • It gives more accurate and precise results than other titrations in which different reagents are used as indicators. That’s why potentiometric titration is preferred over manual titrations. • 1st potentiometric titration was carried out by Robert Behrend in 1893.
- 3. What is potentiometric titration? • Potentiometric titration is a technique similar to direct titration, but in this, no indicator reagent is used; instead, an electrode is used as an indicator. • In potentiometric titrations, a cell is used with a reference electrode, salt bridge, analyte and an indicator electrode. • Generally, the electrolyte solution is used as an analyte. Hydrogen electrodes, silver chloride electrodes and calomel electrodes are generally used as reference electrodes. • Indicator electrode is generally glass electrode and metal ion electrode.
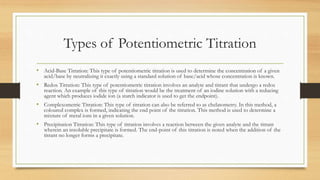
- 4. Types of Potentiometric Titration • Acid-Base Titration: This type of potentiometric titration is used to determine the concentration of a given acid/base by neutralizing it exactly using a standard solution of base/acid whose concentration is known. • Redox Titration: This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. An example of this type of titration would be the treatment of an iodine solution with a reducing agent which produces iodide ion (a starch indicator is used to get the endpoint). • Complexometric Titration: This type of titration can also be referred to as chelatometry. In this method, a coloured complex is formed, indicating the end point of the titration. This method is used to determine a mixture of metal ions in a given solution. • Precipitation Titration: This type of titration involves a reaction between the given analyte and the titrant wherein an insoluble precipitate is formed. The end-point of this titration is noted when the addition of the titrant no longer forms a precipitate.
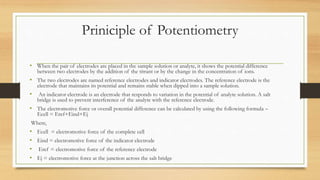
- 9. Priniciple of Potentiometry • When the pair of electrodes are placed in the sample solution or analyte, it shows the potential difference between two electrodes by the addition of the titrant or by the change in the concentration of ions. • The two electrodes are named reference electrodes and indicator electrodes. The reference electrode is the electrode that maintains its potential and remains stable when dipped into a sample solution. • An indicator electrode is an electrode that responds to variation in the potential of analyte solution. A salt bridge is used to prevent interference of the analyte with the reference electrode. • The electromotive force or overall potential difference can be calculated by using the following formula – Ecell = Eref+Eind+Ej Where, • Ecell = electromotive force of the complete cell • Eind = electromotive force of the indicator electrode • Eref = electromotive force of the reference electrode • Ej = electromotive force at the junction across the salt bridge
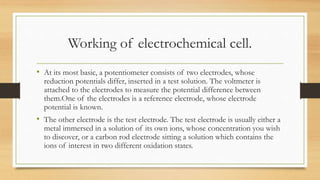
- 10. Working of electrochemical cell. • At its most basic, a potentiometer consists of two electrodes, whose reduction potentials differ, inserted in a test solution. The voltmeter is attached to the electrodes to measure the potential difference between them.One of the electrodes is a reference electrode, whose electrode potential is known. • The other electrode is the test electrode. The test electrode is usually either a metal immersed in a solution of its own ions, whose concentration you wish to discover, or a carbon rod electrode sitting a solution which contains the ions of interest in two different oxidation states.







