preformulation study ralated to pharmaceuticals
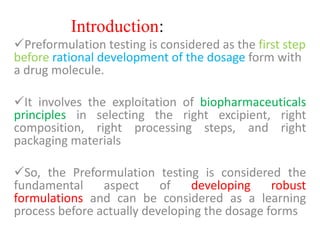
- 2. Introduction: ’ā╝Preformulation testing is considered as the first step before rational development of the dosage form with a drug molecule. ’ā╝It involves the exploitation of biopharmaceuticals principles in selecting the right excipient, right composition, right processing steps, and right packaging materials ’ā╝So, the Preformulation testing is considered the fundamental aspect of developing robust formulations and can be considered as a learning process before actually developing the dosage forms
- 4. Disciplines ’ā╝Medicinal Chemistry and Pharmacology ’ā╝Pre-formulation Research ’ā╝Formulation development ’ā╝Process R&D ’ā╝Analytical R&D ’ā╝Toxicology and drug metabolism
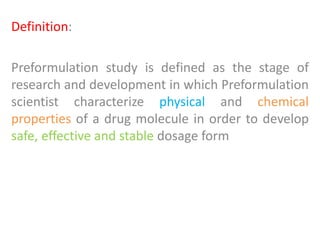
- 5. Definition: Preformulation study is defined as the stage of research and development in which Preformulation scientist characterize physical and chemical properties of a drug molecule in order to develop safe, effective and stable dosage form
- 6. When??? ŌĆó If the drug shows sufficient activity in animals & is to be evaluated in humans. FocusŌĆ”ŌĆ” ŌĆó On physicochemical properties of a new compound which may affect the drug performance &development of efficacious dosage form.
- 7. Objectives: The preformulation investigations confirm that there are no significant barriers to the compoundŌĆÖs development as a marketed drug. The formulation scientist uses these informations to develop dosage forms.
- 8. Goals of Preformulation: ’āśTo generate useful information to the formulator to design an optimum drug delivery system. ’āś To establish the necessary physicochemical parameters of new drug substances. ’āśTo determine kinetic rate profile. ’āśTo establish physical characteristics. ’āśTo establish compatibility with common excipients
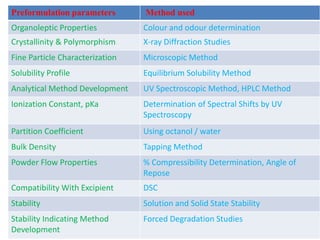
- 10. Preformulation parameters Method used Organoleptic Properties Colour and odour determination Crystallinity & Polymorphism X-ray Diffraction Studies Fine Particle Characterization Microscopic Method Solubility Profile Equilibrium Solubility Method Analytical Method Development UV Spectroscopic Method, HPLC Method Ionization Constant, pKa Determination of Spectral Shifts by UV Spectroscopy Partition Coefficient Using octanol / water Bulk Density Tapping Method Powder Flow Properties % Compressibility Determination, Angle of Repose Compatibility With Excipient DSC Stability Solution and Solid State Stability Stability Indicating Method Development Forced Degradation Studies
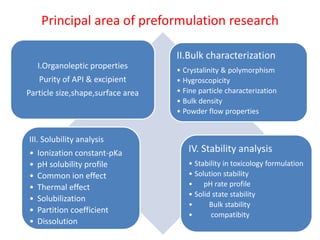
- 11. Principal area of preformulation research I.Organoleptic properties Purity of API & excipient Particle size,shape,surface area II.Bulk characterization ŌĆó Crystalinity & polymorphism ŌĆó Hygroscopicity ŌĆó Fine particle characterization ŌĆó Bulk density ŌĆó Powder flow properties III. Solubility analysis ŌĆó Ionization constant-pKa ŌĆó pH solubility profile ŌĆó Common ion effect ŌĆó Thermal effect ŌĆó Solubilization ŌĆó Partition coefficient ŌĆó Dissolution IV. Stability analysis ŌĆó Stability in toxicology formulation ŌĆó Solution stability ŌĆó pH rate profile ŌĆó Solid state stability ŌĆó Bulk stability ŌĆó compatibity
- 12. Organoleptic properties It refers to the evaluation of drugs by properties like colour, odour, taste An active ingredient must be palatable as well as have a good aroma in case itŌĆÖs not the case then additives like flavours or coating can be done to mask out the taste or hide the intense smell which is otherwise not acceptable. For example: Pungent or sulphur smelling ingredients must be covered with an acceptable odorous compound similarly bland or bitter drugs can be masked for taste.
- 13. Purity of API and excipient ’ā╝These solid drugs are pure organic compounds that exist as either crystalline or amorphous. ’ā╝The purity of the chemical substance is considered as its essential quality to comply with various Pharmacopoeial tests including therapeutic efficacy. ’ā╝Melting point of a chemical substance is considered its intrinsic property which can be used as an indicator of purity of that substance. ’ā╝As an example, a pure crystalline API can be identified by its unique and very sharp melting temperature determined by capillary method. ’ā╝Apart from that method, purity of an API can be determined by HPLC, TLC, DSC, or GC. In chromatographic methods, reference standard of an API is considered 100% pure and unknown samples are compared against that reference standard
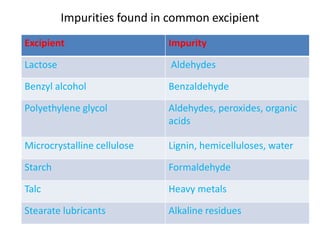
- 14. Impurities found in common excipient Excipient Impurity Lactose Aldehydes Benzyl alcohol Benzaldehyde Polyethylene glycol Aldehydes, peroxides, organic acids Microcrystalline cellulose Lignin, hemicelluloses, water Starch Formaldehyde Talc Heavy metals Stearate lubricants Alkaline residues
- 15. Particle Size, Shape, and Surface Area ’ü▒Certain physical properties, chemical reactivity, stability, bioavailability, content uniformity, sedimentation rate, flow and mixing homogeneity of powders and granules depend on particle size distribution and shape ’ü▒Particle size determination ŌĆó Microscopy.. ŌĆó Anderson Pipette ŌĆó Sieving method ŌĆó Instruments based on light blockage(HIAC) and blockage of electrical conductivity path(coulter Counter) are available
- 16. ’ü▒Shape determination: ŌĆó Microscopy should be carried out to determine the ratio of longest to shortest dimension. It is a shape factor. ’ā╝Surface area determination: The measurement of surface area is made by Brunauer,Emmett,and Teller (BET) nitrogen adsorption ’ā╝By using Scanning electron Microscopy (SEM)
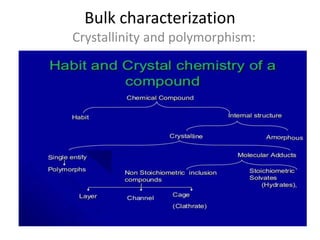
- 17. Bulk characterization Crystallinity and polymorphism:
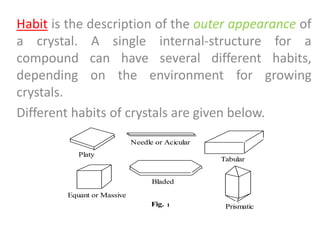
- 18. Habit is the description of the outer appearance of a crystal. A single internal-structure for a compound can have several different habits, depending on the environment for growing crystals. Different habits of crystals are given below. Platy Needle or Acicular Tabular Equant or Massive Bladed Prismatic Fig. 1
- 19. Internal Structure Crystalline state In this state of matter atoms or molecules are arranged in highly ordered form and is associated with three-dimensional array. Amorphous forms In this forms the solids do not have any fixed internal structure. They have atoms or molecules randomly placed as in a liquid. e.g. Amorphous Novobiocin (prepared by rapid precipitation, lyophillization or rapid cooling of molten liquids )
- 20. ’ü▒Since amorphous are usually higher thermodynamic energy than corresponding crystalline forms, solubilities as well as dissolution rates are greater ’ü▒Upon storage amorphous solid tends to revert to more stable form Thermodynamic instability (which occurs during bulk processing or within dosage forms) major disadvantage for developing an amorphous form.
- 21. Preparation Amorphous forms are prepared by rapid precipitation, lyophillization or rapid cooling of molten liquids Glass transition temperature, Tg Tg is a characteristics temperatuer of amorphous form. Below Tg the amorphous form will be brittle and is described as glassy state. Above Tg the solid becomes plastic or rubbery. So Tg is the minimum temperature at which the solid becomes amorphous (plastic) from glassy state.
- 22. Application: ’üČTg can be reduced by addition of plasticizers. Plasticizer molecules, either disturb or distort the molecular arrangements, thus they reduce the Tg. ’üČDuring milling, all the solids must remain below Tg. ’üČAmorphous novobiocin is more soluble and has higher bioavailability than its crystalline form.
- 23. Molecular Adducts During the process of crystallization, some compounds have a tendency to trap the solvent molecules. Non-Stoichiometric inclusion compounds (or adducts) In these crystals solvent molecules are entrapped within the crystal lattice and the number of solvent molecules are not included in stoichiometric number. Usually this adduct is undesirable owing to its lack of reproducibility & should be avoided for development
- 24. Depending on the shape they are of three types :- (1) Channel When the crystal contains continuous channels in which the solvent molecule can be included. e.g . Urea forms channel. (2) Layers:- Here solvent molecules are entrapped in between layers of crystals. (3) Clathrates(Cage):- Solvent molecules are entrapped within the cavity of the crystal from all sides.
- 25. Stoichiometric inclusion compounds (or stoichiometric adducts) This molecular complex has incorporated the crystallizing solvent molecules into specific sites within the crystal lattice and has stoichiometric number of solvent molecules complexed. When the incorporated solvent is water, the complex is called hydrates and when the solvent is other than water, the complex is called solvates. Depending on the ratio of water molecules within a complex the following nomenclature is followed. Anhydrous : 1 mole compound + 0 mole water Hemihydrate: 1 mole compound + ┬Į mole water Monohydrate: 1 mole compound + 1 mole water Dihydrate : 1 mole compound + 2 moles water
- 26. ’ü▒Identification of possible hydrate compounds is important since aqueous solubilities can be significantly less than their anhydrous form ’ü▒Conversion of an anhydrous compound to a hydrate with in the dosage form may reduce the dissolution rate & extent of absorption
- 27. Crystallinity Conversion of an anhydrous compound to a hydrate with in the dosage form may reduce the dissolution rate & extent of absorption ,.houra.,,hourn anhydrous compound to a hydrate within the dosage form may reduce the dissolution rate & extent of drug absorption in the dosage form may reduce the dissolution rate & extent of drug absorption Hours
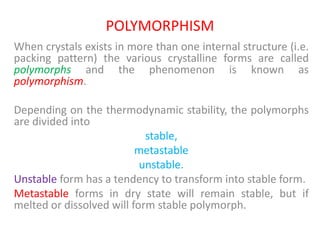
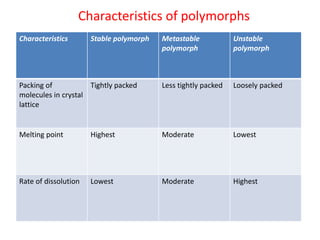
- 28. POLYMORPHISM When crystals exists in more than one internal structure (i.e. packing pattern) the various crystalline forms are called polymorphs and the phenomenon is known as polymorphism. Depending on the thermodynamic stability, the polymorphs are divided into stable, metastable unstable. Unstable form has a tendency to transform into stable form. Metastable forms in dry state will remain stable, but if melted or dissolved will form stable polymorph.
- 29. Characteristics of polymorphs Characteristics Stable polymorph Metastable polymorph Unstable polymorph Packing of molecules in crystal lattice Tightly packed Less tightly packed Loosely packed Melting point Highest Moderate Lowest Rate of dissolution Lowest Moderate Highest
- 30. Polymorphism and bioavailability Many drugs are hydrophobic and have very limited solubility in water. If the drug remains in several polymorphic forms then the stable one will produce the slowest rate of dissolution and it may show minimum bioavailability. For highly water soluble drugs, polymorphism does not show any problem in dissolution rate
- 31. Example: Chloramphenicol palmitate has three polymorphs ╬▒ (stable), ╬▓ (metastable) and ╬│ (unstable). When chloramphenicol palmitate suspension is prepared from ╬▒ or ╬▓ polymorph it is found that bioavailabilty is higher with the metastable form. Example aspirin: Two polymorphs of can be obtained by recrystallization of aspirin from 95% ethanol or n- hexane. The polymorph obtained from n-hexane is found to have greater solubility in water than the polymorph obtained from ethanol.
- 32. Types of polymorphs 1. Enatiotropic (one polymorphs can be reversibly changed into another by varying temp & pressure) (e.g sulfur) 2. Monotropic(one polymorphic form is unstable at all temp & pressure) e.g -glyceryl stearate During preformulation study ,it is importamt to identify stable polymorphs at room temp and determine whether polymorphic transition is possible within the temp range used for stability study and during processing(drying, milling etc.)
- 33. Methods of characterization of polymorphs 1. Hot stage microscopy, 2. Differential Thermal Analysis, 3. Differential Scanning Calorimetry 4. Thermogravimetric Analysis (TGA) 5. X-ray powder diffraction 6. IR-Spectroscopy