SOLID, LIQUID AND GAS science 5 1st quarter.pptx
Download as PPTX, PDF0 likes20 views
solid, liquid and gas science 5
1 of 12
Download to read offline











Recommended
3.1



3.1ambermaine100
╠²
The document discusses the three common states of matter - solids, liquids, and gases. It defines their key properties. Solids have a definite shape and volume, with particles tightly packed in a fixed arrangement. Liquids have a definite volume but no shape, as their particles can flow freely past one another. Gases have neither a definite shape nor volume, with particles spreading apart to fill any available space.states of matter



states of matterParidhi Patel
╠²
The document discusses the states of matter and their properties. It explains that matter can exist in three states - solid, liquid, and gas - depending on the arrangement and movement of particles. In a solid, particles are closely packed in a definite arrangement. In a liquid, particles are closer together but can move past each other. In a gas, particles are far apart and move freely in random directions. The document also discusses the chemical structure of matter, stating that all matter is made up of tiny particles called atoms which have specific masses and properties depending on their element.Solid, liquid and gas behavior as state of matters for students grade 7



Solid, liquid and gas behavior as state of matters for students grade 7NajvaAmin1
╠²
Solid, liquid, and gasThe Kinetic Theory of Matter



The Kinetic Theory of MatterM BR
╠²
The document describes the kinetic theory of matter and the arrangement and movement of particles in the three states of matter - solid, liquid, and gas. In solids, particles are packed closely together in an orderly arrangement with strong forces between them, allowing only vibration and rotation. In liquids, particles are also packed closely but in a less orderly way, with weaker forces allowing vibration, rotation and movement between collisions. Gases have particles very far apart with weak forces, allowing free and rapid vibration, rotation and movement.2. Basic Principle of Particle Nature of Matter.pptx



2. Basic Principle of Particle Nature of Matter.pptxMAnnGeronaLintao
╠²
Basic Principle of Particle Nature of Matter2. Basic Principle of Particle Nature of Matter.pptx



2. Basic Principle of Particle Nature of Matter.pptxMAnnGeronaLintao
╠²
Basic Principle of Particle Nature of MatterClassification of matter and changes



Classification of matter and changesBhavana Goud
╠²
This document discusses the classification and properties of matter. It defines matter as anything that has mass and occupies space, and classifies matter based on its physical state as solid, liquid or gas. Solids have a fixed shape and volume, liquids take the shape of their container but maintain a fixed volume, and gases expand to fill their container. Matter can also be classified based on its composition - pure substances have a uniform composition, while mixtures are combinations of two or more substances. Elements are pure substances made of a single type of atom, while compounds are pure substances made of molecules containing two or more elements. The document distinguishes between physical changes, which alter a substance's state or properties without changing its composition, and chemical changes,Kinetic Particle Theory (Slg Introduction)



Kinetic Particle Theory (Slg Introduction)sYhira
╠²
This document discusses kinetic particle theory and the three states of matter - solids, liquids, and gases. It describes the arrangement and movement of particles in each state and how they differ. Specifically, it explains that in solids, particles are closely packed in an orderly arrangement and vibrate about fixed positions. In liquids, particles are closely packed disorderly and can move throughout while in gases, particles are far apart and move randomly. The document also covers how states are determined by intermolecular forces and particle energy.States of Matter Power Point and stages o



States of Matter Power Point and stages oCPTiwari3
╠²
Physics - state of matter definition and, variod staged and detailed
This report is sent to the certification body or bodies, the members of the audit team and the audit representative of the organisation. All documents (such as this report) regarding the certification procedure are treated confidentially by the audit team and the certification body. This audit report remains the property of the certification body.
An audit is a procedure based on the principle of random sampling and cannot cover each detail of the management system. Therefore nonconformities of weaknesses may still exist which were not expressly mentioned by the auditors in the final meeting or in the audit report.
The responsibility for continuous effective operation of the management system always rests solely with the audited and certified organisation.
Salvo clause:
The audit report will be left to the organisation at the end of the audit - subject to approval by the certification body. The independent veto process may cause modifications or additions. In these cases a modified revision will be sent to the audited organisation.
In any regular audit the audit team shall see and review the following objective evidences.
To confirm, the corresponding revision information is registered in column ŌĆ×EditionŌĆ£
That can become applicable as well for some or all the listed objectives in special audits, e.g. for extensions or after transferring sites.
At least in initial/recertification or extension audits (or when necessary) these objective evidences/documents are attached adequately to the audit file and uploaded into the release workflow.
In any other audit it is accepted to record the revision information only.
Standard-specific evidence, as applicable (e.g. ISO 14001: extract of environmental permit register; ISO 27001: statement of applicability, ISO 45001: accident statistics; ISO 50001: energy report as cover sheet with date and signature or evidence of continual energy performance improvement)
Taking into account the audit findings documented below, the organisation's size and structure, objectives, scope of the management system, processes and results achieved, the organisation has demonstrated that it operates its management system to ensure conformity with its own requirements, the requirements of interested parties, applicable legal requirements and appropriate requirements from the management system standards.Identified nonconformities are each documented in a nonconformity report ("Management of a nonconformity"), which are part of this audit report as annexes.
The audit team uses the nonconformity reports after the audit to track the processing status and also documents in them the final assessment results for the nonconformities concerned.
The organization shall perform a root cause analysis for any nonconformity and define adequate corrective actions. Root cause analysis, corrective actions including action plan for implementation and - if applicable- objectSTATES OF MATTER



STATES OF MATTERMadihaAsad5
╠²
States of matter include solids, liquids, gases, and plasma. Solids have a definite shape and volume, with particles tightly packed and exhibiting vibrational motion. Liquids take the shape of their container but have a definite volume, with particles able to flow steadily and slide past one another. Gases have no definite shape or volume, with particles having translational, rotational, and vibrational motion and the ability to diffuse freely. The three common states differ in terms of compressibility, density, intermolecular forces, and particle behavior.L1-PROPERTIES-OF-MATTER.pptx



L1-PROPERTIES-OF-MATTER.pptxKeithLabustro
╠²
This document contains a prayer asking God for blessings on a new day of study, including a witty brain, inquisitive mind, and wisdom to follow God's commandments. It also asks God to bless students' homes so they can use their studies for daily life and service to God's holy name. The prayer is signed in the name of Jesus Christ. The document then provides rules and an activity for an online science class, including unscrambling terms related to properties of matter and checking answers to true/false questions about these properties.CHEMISTRY GEN CHEM 2 Kinetic-Molecular-Model.pptx



CHEMISTRY GEN CHEM 2 Kinetic-Molecular-Model.pptxNoreenzelJoyVillanue
╠²
Kinetic molecular model GEN CHEM 2Matter (1).pdf



Matter (1).pdfHimemLizardo
╠²
Matter is anything that occupies space and has mass. It exists in various states depending on the behavior and arrangement of its atoms and molecules. The three main states of matter are solids, liquids, and gases. Solids have a fixed shape and volume, with atoms closely bonded in a rigid structure. Liquids have a fixed volume but can change shape, as atoms are loosely bonded and free to move around one another. Gases have no fixed shape or volume, as atoms are very far apart and move freely in all directions. The states can change from one to another when sufficient heat is added or removed without altering the chemical makeup of the substance.States of Matter .pptx



States of Matter .pptxLearningwithNasirKha
╠²
║▌║▌▀Ż 1: Introduction
Let's start by understanding what "matter" is. Matter is anything that has mass and takes up space. Imagine your favorite toys, water, and the air around you - they're all forms of matter!
║▌║▌▀Ż 2: Solids: Think of your toys, books, and even ice cream! Solids have a fixed shape and volume.
║▌║▌▀Ż 3:Liquids: Pour yourself a glass of juice. Liquids take the shape of their container but have a constant volume.
║▌║▌▀Ż 4:Gases: Imagine the air you breathe or the helium in balloons. Gases have neither a fixed shape nor a fixed volume.
║▌║▌▀Ż 5: Quick RecapMatter(notes).pptx



Matter(notes).pptxssuser26eb5b
╠²
This document discusses the classification of matter. It defines matter as substances that have mass and volume and occupy space. Matter is made up of tiny particles called atoms and molecules. The characteristics of these particles determine the three states of matter: solid, liquid, and gas. Solids have a definite shape and volume, do not flow, and are rigid and incompressible. Liquids have a definite volume but take the shape of their container, and can flow and diffuse. Gases have no definite shape or volume, and will expand to fill their container. Non-matter substances have no mass or volume and are examples of energy or abstract concepts.Matters



MattersLesli Sombilon
╠²
The document discusses the different states of matter and phases. It explains that matter can exist in solid, liquid, gas, and plasma phases. The solid phase occurs when molecules are tightly bound, while the liquid phase allows molecules to flow freely but maintain a shape defined by their container. Gas molecules exhibit random motion and the least intermolecular forces. Plasma is an ionized gas that conducts electricity. The document also briefly discusses Bose-Einstein condensates, an exotic state of matter.Properties of matter



Properties of matterNeilfieOrit2
╠²
Matter is anything that takes up space and has mass. It exists in three states: solid, liquid, and gas. Solids have a definite shape and volume as their particles are tightly packed and vibrate in fixed positions. Liquids have an indefinite shape but definite volume as their particles are closely packed but can slide over one another. Gases have an indefinite shape and volume as their particles are very far apart and move freely. Physical properties can be observed without changing the chemical makeup and include color, texture, temperature, mass, volume, shape, size, weight, and ability to flow.Phases of Matter



Phases of MatterRobert Joshua Busalla
╠²
There are four fundamental forms of matter: solids, liquids, gases, and plasma. Solids have a definite shape and volume due to their rigid molecular structure. Liquids also have a definite volume but cannot maintain a definite shape as their molecules are drawn together weakly, allowing liquids to flow and take the shape of their container. Gases have neither a definite shape nor volume as their molecules are very weakly bonded and spread out to fill their container. Plasma differs from solids, liquids, and gases in that its molecules are ionized, meaning they are positively or negatively charged, and thus exhibit electromagnetic properties.FLEX-Particle nature of matter.pptx



FLEX-Particle nature of matter.pptxSHIELABALINGCOS2
╠²
The document discusses different topics relating to earth science including the relationship between faults and earthquakes, typhoons in the Philippines, the differences between meteoroid, meteor and meteorite, and the properties of comets and asteroids. It also covers states of matter and concepts about particles, including how solids, liquids, and gases differ at the particle level. Several hands-on activities are presented to help explain these concepts.Grade 7A - State of Matter - Liquid Group 6



Grade 7A - State of Matter - Liquid Group 6Kamille Gabayno
╠²
This science presentation discusses the properties of liquids and how they change states. It notes that liquids have a definite volume but no fixed shape, as their particles can move around one another. The presentation explains that in liquids, particles have no fixed positions but are held closer together than in gases by intermolecular forces. It also shows diagrams of how particles are arranged in liquids, with space between them but still closer than in gases.Matter(arrangement of molecules



Matter(arrangement of moleculesDivya Kumar
╠²
The document discusses molecules and their properties. It defines a molecule as the smallest particle of a substance that retains the properties of that substance. Molecules are made up of atoms and their arrangement differs between solids, liquids, and gases. In solids, molecules are closely packed with strong attractive forces, giving solids a definite shape and volume. In liquids, molecules are less tightly packed with moderate attractive forces, allowing liquids to flow and take the shape of their container while maintaining a definite volume. In gases, molecules are loosely packed with weak attractive forces, causing gases to expand freely without a definite shape or volume.Pink and Green Doodle Hand drawn Science Project Presentation.pptx



Pink and Green Doodle Hand drawn Science Project Presentation.pptxMaryAnnFrias3
╠²
pink and green doodlethree states of matter..................pptx



three states of matter..................pptxnorthernsamarjbbinam
╠²
Solids, liquids, and gases are the three main states of matter. Solids have a definite shape and volume, do not flow, and are generally incompressible. Liquids have a definite volume but no definite shape, flow slowly, and are almost incompressible. Gases have no definite shape or volume, flow and diffuse easily, and are compressible. The arrangement and movement of particles determines the properties of each state.Presentation of science (matter)



Presentation of science (matter)Kunnu Aggarwal
╠²
Matter is anything that has mass and occupies space. It exists in three main states: solid, liquid, and gas. The state depends on how tightly or loosely the particles are packed. Solids have a fixed shape and volume as particles are tightly packed and vibrate in place. Liquids take the shape of their container but maintain a fixed volume as particles can move past one another. Gases have no definite shape or volume as particles are very far apart and move freely. Water can change states by adding or removing heat, going from solid ice to liquid to gas vapor.5 physical properties of pharmaceutical material - copy



5 physical properties of pharmaceutical material - copyUniversity of Zambia, School of Pharmacy, Lusaka, Zambia
╠²
This document defines key terms and concepts related to the properties and classification of matter. It discusses the characteristics of matter such as mass, inertia, weight, density and gravity. It describes the three states of matter - solids, liquids, and gases - and classifies matter as either pure substances or mixtures. The document also distinguishes between physical properties, which can be observed without changing the identity of a substance, and chemical properties, which result in a new substance. Key concepts covered include phases of matter, viscosity, surface tension, phase changes, and the differences between physical and chemical changes. Interactive questions are provided to illustrate these fundamental chemistry concepts.GROUP-4-DEVELOPMENT-OF-CLASSROOM-ASSESSMENT-FOR-MEASURING-KNOWLEDGE-LEARNING-...



GROUP-4-DEVELOPMENT-OF-CLASSROOM-ASSESSMENT-FOR-MEASURING-KNOWLEDGE-LEARNING-...kathtolentino55
╠²
assessment
More Related Content
Similar to SOLID, LIQUID AND GAS science 5 1st quarter.pptx (20)
States of Matter Power Point and stages o



States of Matter Power Point and stages oCPTiwari3
╠²
Physics - state of matter definition and, variod staged and detailed
This report is sent to the certification body or bodies, the members of the audit team and the audit representative of the organisation. All documents (such as this report) regarding the certification procedure are treated confidentially by the audit team and the certification body. This audit report remains the property of the certification body.
An audit is a procedure based on the principle of random sampling and cannot cover each detail of the management system. Therefore nonconformities of weaknesses may still exist which were not expressly mentioned by the auditors in the final meeting or in the audit report.
The responsibility for continuous effective operation of the management system always rests solely with the audited and certified organisation.
Salvo clause:
The audit report will be left to the organisation at the end of the audit - subject to approval by the certification body. The independent veto process may cause modifications or additions. In these cases a modified revision will be sent to the audited organisation.
In any regular audit the audit team shall see and review the following objective evidences.
To confirm, the corresponding revision information is registered in column ŌĆ×EditionŌĆ£
That can become applicable as well for some or all the listed objectives in special audits, e.g. for extensions or after transferring sites.
At least in initial/recertification or extension audits (or when necessary) these objective evidences/documents are attached adequately to the audit file and uploaded into the release workflow.
In any other audit it is accepted to record the revision information only.
Standard-specific evidence, as applicable (e.g. ISO 14001: extract of environmental permit register; ISO 27001: statement of applicability, ISO 45001: accident statistics; ISO 50001: energy report as cover sheet with date and signature or evidence of continual energy performance improvement)
Taking into account the audit findings documented below, the organisation's size and structure, objectives, scope of the management system, processes and results achieved, the organisation has demonstrated that it operates its management system to ensure conformity with its own requirements, the requirements of interested parties, applicable legal requirements and appropriate requirements from the management system standards.Identified nonconformities are each documented in a nonconformity report ("Management of a nonconformity"), which are part of this audit report as annexes.
The audit team uses the nonconformity reports after the audit to track the processing status and also documents in them the final assessment results for the nonconformities concerned.
The organization shall perform a root cause analysis for any nonconformity and define adequate corrective actions. Root cause analysis, corrective actions including action plan for implementation and - if applicable- objectSTATES OF MATTER



STATES OF MATTERMadihaAsad5
╠²
States of matter include solids, liquids, gases, and plasma. Solids have a definite shape and volume, with particles tightly packed and exhibiting vibrational motion. Liquids take the shape of their container but have a definite volume, with particles able to flow steadily and slide past one another. Gases have no definite shape or volume, with particles having translational, rotational, and vibrational motion and the ability to diffuse freely. The three common states differ in terms of compressibility, density, intermolecular forces, and particle behavior.L1-PROPERTIES-OF-MATTER.pptx



L1-PROPERTIES-OF-MATTER.pptxKeithLabustro
╠²
This document contains a prayer asking God for blessings on a new day of study, including a witty brain, inquisitive mind, and wisdom to follow God's commandments. It also asks God to bless students' homes so they can use their studies for daily life and service to God's holy name. The prayer is signed in the name of Jesus Christ. The document then provides rules and an activity for an online science class, including unscrambling terms related to properties of matter and checking answers to true/false questions about these properties.CHEMISTRY GEN CHEM 2 Kinetic-Molecular-Model.pptx



CHEMISTRY GEN CHEM 2 Kinetic-Molecular-Model.pptxNoreenzelJoyVillanue
╠²
Kinetic molecular model GEN CHEM 2Matter (1).pdf



Matter (1).pdfHimemLizardo
╠²
Matter is anything that occupies space and has mass. It exists in various states depending on the behavior and arrangement of its atoms and molecules. The three main states of matter are solids, liquids, and gases. Solids have a fixed shape and volume, with atoms closely bonded in a rigid structure. Liquids have a fixed volume but can change shape, as atoms are loosely bonded and free to move around one another. Gases have no fixed shape or volume, as atoms are very far apart and move freely in all directions. The states can change from one to another when sufficient heat is added or removed without altering the chemical makeup of the substance.States of Matter .pptx



States of Matter .pptxLearningwithNasirKha
╠²
║▌║▌▀Ż 1: Introduction
Let's start by understanding what "matter" is. Matter is anything that has mass and takes up space. Imagine your favorite toys, water, and the air around you - they're all forms of matter!
║▌║▌▀Ż 2: Solids: Think of your toys, books, and even ice cream! Solids have a fixed shape and volume.
║▌║▌▀Ż 3:Liquids: Pour yourself a glass of juice. Liquids take the shape of their container but have a constant volume.
║▌║▌▀Ż 4:Gases: Imagine the air you breathe or the helium in balloons. Gases have neither a fixed shape nor a fixed volume.
║▌║▌▀Ż 5: Quick RecapMatter(notes).pptx



Matter(notes).pptxssuser26eb5b
╠²
This document discusses the classification of matter. It defines matter as substances that have mass and volume and occupy space. Matter is made up of tiny particles called atoms and molecules. The characteristics of these particles determine the three states of matter: solid, liquid, and gas. Solids have a definite shape and volume, do not flow, and are rigid and incompressible. Liquids have a definite volume but take the shape of their container, and can flow and diffuse. Gases have no definite shape or volume, and will expand to fill their container. Non-matter substances have no mass or volume and are examples of energy or abstract concepts.Matters



MattersLesli Sombilon
╠²
The document discusses the different states of matter and phases. It explains that matter can exist in solid, liquid, gas, and plasma phases. The solid phase occurs when molecules are tightly bound, while the liquid phase allows molecules to flow freely but maintain a shape defined by their container. Gas molecules exhibit random motion and the least intermolecular forces. Plasma is an ionized gas that conducts electricity. The document also briefly discusses Bose-Einstein condensates, an exotic state of matter.Properties of matter



Properties of matterNeilfieOrit2
╠²
Matter is anything that takes up space and has mass. It exists in three states: solid, liquid, and gas. Solids have a definite shape and volume as their particles are tightly packed and vibrate in fixed positions. Liquids have an indefinite shape but definite volume as their particles are closely packed but can slide over one another. Gases have an indefinite shape and volume as their particles are very far apart and move freely. Physical properties can be observed without changing the chemical makeup and include color, texture, temperature, mass, volume, shape, size, weight, and ability to flow.Phases of Matter



Phases of MatterRobert Joshua Busalla
╠²
There are four fundamental forms of matter: solids, liquids, gases, and plasma. Solids have a definite shape and volume due to their rigid molecular structure. Liquids also have a definite volume but cannot maintain a definite shape as their molecules are drawn together weakly, allowing liquids to flow and take the shape of their container. Gases have neither a definite shape nor volume as their molecules are very weakly bonded and spread out to fill their container. Plasma differs from solids, liquids, and gases in that its molecules are ionized, meaning they are positively or negatively charged, and thus exhibit electromagnetic properties.FLEX-Particle nature of matter.pptx



FLEX-Particle nature of matter.pptxSHIELABALINGCOS2
╠²
The document discusses different topics relating to earth science including the relationship between faults and earthquakes, typhoons in the Philippines, the differences between meteoroid, meteor and meteorite, and the properties of comets and asteroids. It also covers states of matter and concepts about particles, including how solids, liquids, and gases differ at the particle level. Several hands-on activities are presented to help explain these concepts.Grade 7A - State of Matter - Liquid Group 6



Grade 7A - State of Matter - Liquid Group 6Kamille Gabayno
╠²
This science presentation discusses the properties of liquids and how they change states. It notes that liquids have a definite volume but no fixed shape, as their particles can move around one another. The presentation explains that in liquids, particles have no fixed positions but are held closer together than in gases by intermolecular forces. It also shows diagrams of how particles are arranged in liquids, with space between them but still closer than in gases.Matter(arrangement of molecules



Matter(arrangement of moleculesDivya Kumar
╠²
The document discusses molecules and their properties. It defines a molecule as the smallest particle of a substance that retains the properties of that substance. Molecules are made up of atoms and their arrangement differs between solids, liquids, and gases. In solids, molecules are closely packed with strong attractive forces, giving solids a definite shape and volume. In liquids, molecules are less tightly packed with moderate attractive forces, allowing liquids to flow and take the shape of their container while maintaining a definite volume. In gases, molecules are loosely packed with weak attractive forces, causing gases to expand freely without a definite shape or volume.Pink and Green Doodle Hand drawn Science Project Presentation.pptx



Pink and Green Doodle Hand drawn Science Project Presentation.pptxMaryAnnFrias3
╠²
pink and green doodlethree states of matter..................pptx



three states of matter..................pptxnorthernsamarjbbinam
╠²
Solids, liquids, and gases are the three main states of matter. Solids have a definite shape and volume, do not flow, and are generally incompressible. Liquids have a definite volume but no definite shape, flow slowly, and are almost incompressible. Gases have no definite shape or volume, flow and diffuse easily, and are compressible. The arrangement and movement of particles determines the properties of each state.Presentation of science (matter)



Presentation of science (matter)Kunnu Aggarwal
╠²
Matter is anything that has mass and occupies space. It exists in three main states: solid, liquid, and gas. The state depends on how tightly or loosely the particles are packed. Solids have a fixed shape and volume as particles are tightly packed and vibrate in place. Liquids take the shape of their container but maintain a fixed volume as particles can move past one another. Gases have no definite shape or volume as particles are very far apart and move freely. Water can change states by adding or removing heat, going from solid ice to liquid to gas vapor.5 physical properties of pharmaceutical material - copy



5 physical properties of pharmaceutical material - copyUniversity of Zambia, School of Pharmacy, Lusaka, Zambia
╠²
This document defines key terms and concepts related to the properties and classification of matter. It discusses the characteristics of matter such as mass, inertia, weight, density and gravity. It describes the three states of matter - solids, liquids, and gases - and classifies matter as either pure substances or mixtures. The document also distinguishes between physical properties, which can be observed without changing the identity of a substance, and chemical properties, which result in a new substance. Key concepts covered include phases of matter, viscosity, surface tension, phase changes, and the differences between physical and chemical changes. Interactive questions are provided to illustrate these fundamental chemistry concepts.5 physical properties of pharmaceutical material - copy



5 physical properties of pharmaceutical material - copyUniversity of Zambia, School of Pharmacy, Lusaka, Zambia
╠²
More from kathtolentino55 (20)
GROUP-4-DEVELOPMENT-OF-CLASSROOM-ASSESSMENT-FOR-MEASURING-KNOWLEDGE-LEARNING-...



GROUP-4-DEVELOPMENT-OF-CLASSROOM-ASSESSMENT-FOR-MEASURING-KNOWLEDGE-LEARNING-...kathtolentino55
╠²
assessment
Recently uploaded (20)
APM People Interest Network Conference - Oliver Randall & David Bovis - Own Y...



APM People Interest Network Conference - Oliver Randall & David Bovis - Own Y...Association for Project Management
╠²
APM People Interest Network Conference 2025
- Autonomy, Teams and Tension
- Oliver Randall & David Bovis
- Own Your Autonomy
Oliver Randall
Consultant, Tribe365
Oliver is a career project professional since 2011 and started volunteering with APM in 2016 and has since chaired the People Interest Network and the North East Regional Network. Oliver has been consulting in culture, leadership and behaviours since 2019 and co-developed HPTM┬«ŌĆ»an off the shelf high performance framework for teams and organisations and is currently working with SAS (Stellenbosch Academy for Sport) developing the culture, leadership and behaviours framework for future elite sportspeople whilst also holding down work as a project manager in the NHS at North Tees and Hartlepool Foundation Trust.
David Bovis
Consultant, Duxinaroe
A Leadership and Culture Change expert, David is the originator of BTFAŌäó and The Dux Model.
With a Masters in Applied Neuroscience from the Institute of Organisational Neuroscience, he is widely regarded as the ŌĆśGo-ToŌĆÖ expert in the field, recognised as an inspiring keynote speaker and change strategist.
He has an industrial engineering background, majoring in TPS / Lean. David worked his way up from his apprenticeship to earn his seat at the C-suite table. His career spans several industries, including Automotive, Aerospace, Defence, Space, Heavy Industries and Elec-Mech / polymer contract manufacture.
Published in LondonŌĆÖs Evening Standard quarterly business supplement, James CaanŌĆÖs ŌĆśYour businessŌĆÖ Magazine, ŌĆśQuality WorldŌĆÖ, the Lean Management Journal and Cambridge Universities ŌĆśPMAŌĆÖ, he works as comfortably with leaders from FTSE and Fortune 100 companies as he does owner-managers in SMEŌĆÖs. He is passionate about helping leaders understand the neurological root cause of a high-performance culture and sustainable change, in business.
Session | Own Your Autonomy ŌĆō The Importance of Autonomy in Project Management
#OwnYourAutonomy is aiming to be a global APM initiative to position everyone to take a more conscious role in their decision making process leading to increased outcomes for everyone and contribute to ŌĆ£a world in which all projects succeedŌĆØ.
We want everyone to join the journey.
#OwnYourAutonomy is the culmination of 3 years of collaborative exploration within the Leadership Focus Group which is part of the APM People Interest Network. The work has been pulled together using the 5 HPTM® Systems and the BTFA neuroscience leadership programme.
https://www.linkedin.com/showcase/apm-people-network/about/APM People Interest Network Conference - Tim Lyons - The neurological levels ...



APM People Interest Network Conference - Tim Lyons - The neurological levels ...Association for Project Management
╠²
APM People Interest Network Conference 2025
-Autonomy, Teams and Tension: Projects under stress
-Tim Lyons
-The neurological levels of
team-working: Harmony and tensions
With a background in projects spanning more than 40 years, Tim Lyons specialised in the delivery of large, complex, multi-disciplinary programmes for clients including Crossrail, Network Rail, ExxonMobil, Siemens and in patent development. His first career was in broadcasting, where he designed and built commercial radio station studios in Manchester, Cardiff and Bristol, also working as a presenter and programme producer. Tim now writes and presents extensively on matters relating to the human and neurological aspects of projects, including communication, ethics and coaching. He holds a MasterŌĆÖs degree in NLP, is an NLP Master Practitioner and International Coach. He is the Deputy Lead for APMŌĆÖs People Interest Network.
Session | The Neurological Levels of Team-working: Harmony and Tensions
Understanding how teams really work at conscious and unconscious levels is critical to a harmonious workplace. This session uncovers what those levels are, how to use them to detect and avoid tensions and how to smooth the management of change by checking you have considered all of them.Database population in Odoo 18 - Odoo slides



Database population in Odoo 18 - Odoo slidesCeline George
╠²
In this slide, weŌĆÖll discuss the database population in Odoo 18. In Odoo, performance analysis of the source code is more important. Database population is one of the methods used to analyze the performance of our code. How to use Init Hooks in Odoo 18 - Odoo ║▌║▌▀Żs



How to use Init Hooks in Odoo 18 - Odoo ║▌║▌▀ŻsCeline George
╠²
In this slide, weŌĆÖll discuss on how to use Init Hooks in Odoo 18. In Odoo, Init Hooks are essential functions specified as strings in the __init__ file of a module.TLE 7 - 3rd Topic - Hand Tools, Power Tools, Instruments, and Equipment Used ...



TLE 7 - 3rd Topic - Hand Tools, Power Tools, Instruments, and Equipment Used ...RizaBedayo
╠²
Hand Tools, Power Tools, and Equipment in Industrial ArtsThe Battle of Belgrade Road: A WW1 Street Renaming Saga by Amir Dotan



The Battle of Belgrade Road: A WW1 Street Renaming Saga by Amir DotanHistory of Stoke Newington
╠²
Presented at the 24th Stoke Newington History Talks event on 27th Feb 2025
https://stokenewingtonhistory.com/stoke-newington-history-talks/Essentials of a Good PMO, presented by Aalok Sonawala



Essentials of a Good PMO, presented by Aalok SonawalaAssociation for Project Management
╠²
APM event hosted by the South Wales and West of England Network (SWWE Network)
Speaker: Aalok Sonawala
The SWWE Regional Network were very pleased to welcome Aalok Sonawala, Head of PMO, National Programmes, Rider Levett Bucknall on 26 February, to BAWA for our first face to face event of 2025. Aalok is a member of APMŌĆÖs Thames Valley Regional Network and also speaks to members of APMŌĆÖs PMO Interest Network, which aims to facilitate collaboration and learning, offer unbiased advice and guidance.
Tonight, Aalok planned to discuss the importance of a PMO within project-based organisations, the different types of PMO and their key elements, PMO governance and centres of excellence.
PMOŌĆÖs within an organisation can be centralised, hub and spoke with a central PMO with satellite PMOs globally, or embedded within projects. The appropriate structure will be determined by the specific business needs of the organisation. The PMO sits above PM delivery and the supply chain delivery teams.
For further information about the event please click here.A PPT Presentation on The Princess and the God: A tale of ancient India by A...



A PPT Presentation on The Princess and the God: A tale of ancient India by A...Beena E S
╠²
A PPT Presentation on The Princess and the God: A tale of ancient India by Aaron ShepardComputer Network Unit IV - Lecture Notes - Network Layer



Computer Network Unit IV - Lecture Notes - Network LayerMurugan146644
╠²
Title:
Lecture Notes - Unit IV - The Network Layer
Description:
Welcome to the comprehensive guide on Computer Network concepts, tailored for final year B.Sc. Computer Science students affiliated with Alagappa University. This document covers fundamental principles and advanced topics in Computer Network. PDF content is prepared from the text book Computer Network by Andrew S. Tenanbaum
Key Topics Covered:
Main Topic : The Network Layer
Sub-Topic : Network Layer Design Issues (Store and forward packet switching , service provided to the transport layer, implementation of connection less service, implementation of connection oriented service, Comparision of virtual circuit and datagram subnet), Routing algorithms (Shortest path routing, Flooding , Distance Vector routing algorithm, Link state routing algorithm , hierarchical routing algorithm, broadcast routing, multicast routing algorithm)
Other Link :
1.Introduction to computer network - /slideshow/lecture-notes-introduction-to-computer-network/274183454
2. Physical Layer - /slideshow/lecture-notes-unit-ii-the-physical-layer/274747125
3. Data Link Layer Part 1 : /slideshow/lecture-notes-unit-iii-the-datalink-layer/275288798
Target Audience:
Final year B.Sc. Computer Science students at Alagappa University seeking a solid foundation in Computer Network principles for academic.
About the Author:
Dr. S. Murugan is Associate Professor at Alagappa Government Arts College, Karaikudi. With 23 years of teaching experience in the field of Computer Science, Dr. S. Murugan has a passion for simplifying complex concepts in Computer Network
Disclaimer:
This document is intended for educational purposes only. The content presented here reflects the authorŌĆÖs understanding in the field of Computer NetworkHow to attach file using upload button Odoo 18



How to attach file using upload button Odoo 18Celine George
╠²
In this slide, weŌĆÖll discuss on how to attach file using upload button Odoo 18. Odoo features a dedicated model, 'ir.attachments,' designed for storing attachments submitted by end users. We can see the process of utilizing the 'ir.attachments' model to enable file uploads through web forms in this slide.Adventure Activities Final By H R Gohil Sir



Adventure Activities Final By H R Gohil SirGUJARATCOMMERCECOLLE
╠²
Adventure Activities Final By H R Gohil SirSouth Hornsey: The Lost Local Authority that Merged with Stoke Newington by T...



South Hornsey: The Lost Local Authority that Merged with Stoke Newington by T...History of Stoke Newington
╠²
Presented at the 24th Stoke Newington History Talks event on 27th Feb 2025
https://stokenewingtonhistory.com/stoke-newington-history-talks/How to Manage Putaway Rule in Odoo 17 Inventory



How to Manage Putaway Rule in Odoo 17 InventoryCeline George
╠²
Inventory management is a critical aspect of any business involved in manufacturing or selling products.
Odoo 17 offers a robust inventory management system that can handle complex operations and optimize warehouse efficiency. How to Setup WhatsApp in Odoo 17 - Odoo ║▌║▌▀Żs



How to Setup WhatsApp in Odoo 17 - Odoo ║▌║▌▀ŻsCeline George
╠²
Integrate WhatsApp into Odoo using the WhatsApp Business API or third-party modules to enhance communication. This integration enables automated messaging and customer interaction management within Odoo 17.FESTIVAL: SINULOG & THINGYAN-LESSON 4.pptx



FESTIVAL: SINULOG & THINGYAN-LESSON 4.pptxDanmarieMuli1
╠²
Sinulog Festival of Cebu City, and Thingyan Festival of Myanmar.Mate, a short story by Kate Grenvile.pptx



Mate, a short story by Kate Grenvile.pptxLiny Jenifer
╠²
A powerpoint presentation on the short story Mate by Kate Greenville. This presentation provides information on Kate Greenville, a character list, plot summary and critical analysis of the short story.APM People Interest Network Conference - Oliver Randall & David Bovis - Own Y...



APM People Interest Network Conference - Oliver Randall & David Bovis - Own Y...Association for Project Management
╠²
APM People Interest Network Conference - Tim Lyons - The neurological levels ...



APM People Interest Network Conference - Tim Lyons - The neurological levels ...Association for Project Management
╠²
South Hornsey: The Lost Local Authority that Merged with Stoke Newington by T...



South Hornsey: The Lost Local Authority that Merged with Stoke Newington by T...History of Stoke Newington
╠²
SOLID, LIQUID AND GAS science 5 1st quarter.pptx
- 2. A property of matter ’üĄA property of matter describes how an object looks, feels, smells, reacts It differentiated solid, liquid, and gas from one another. Knowing their properties is the best way to identify and distinguish the states of matter.
- 3. SOLID ’üĄSolid materials have particles (atoms or molecules) that are tightly connected with one another. Since these particles are very attracted to one another, they vibrate but do not move. This is how solid objects retain their shape unless something is done to deliberately change them. Solid materials also have a definite volume.
- 4. SOLID
- 5. LIQUID ’üĄThe attraction of molecules in liquids is weaker compared to that in solids. The particles vibrate, move more freely, and slide past one another. Because of this, liquid materials do not have their own shape. They only take the shape of their container.
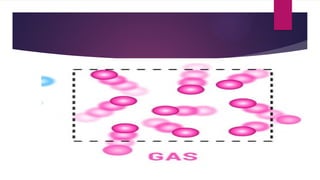
- 7. GAS ’üĄThe particles of gases are dispersed randomly. These particles have the weakest attraction to one another as compared to the attraction between particles of the other states of matter. The molecules of gases move constantly in an unsystematic way. Hence, it is hard to identify their shape. Gases can fill up an entire container.

























