Stephen 4.31.doc
Download as doc, pdf0 likes12 views
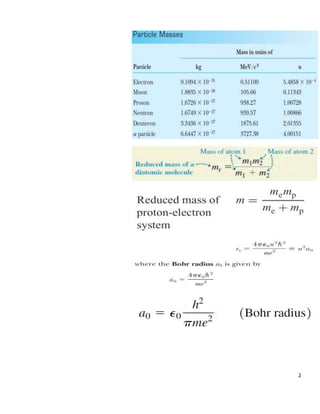
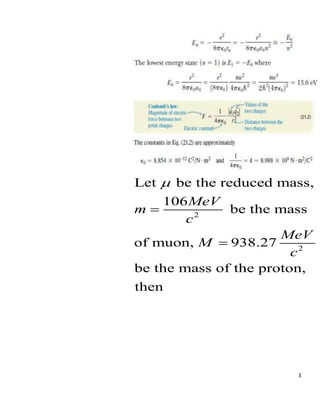
A muonic atom consists of a muon in place of an electron orbiting the nucleus. For a muon in a hydrogen atom: (a) The smallest radius of the muon orbit is 151.46 femtometers. (b) The binding energy of the muon is 2518 electronvolts. The reduced mass and Bohr model were used to calculate the radius and binding energy from the muon and proton masses.
1 of 8
Download to read offline






Ad
Recommended
Electrodynamics & Electrical Technology dated 27-06-2024
Electrodynamics & Electrical Technology dated 27-06-2024Rajput AbdulWaheed Bhatti
╠²
Physics Collection 27-06-2024Electronics & Electrodynamics NOTES dated 27-06-2024
Electronics & Electrodynamics NOTES dated 27-06-2024Rajput AbdulWaheed Bhatti
╠²
The document contains a sequential list of numbers from 1 to 66. It appears to be a simple enumeration without any additional context. There are no further details provided regarding the significance or purpose of the numbers.Electrodynamics Notes Young Freedman and Richard Wolfson
Electrodynamics Notes Young Freedman and Richard WolfsonRajput AbdulWaheed Bhatti
╠²
The document consists of a numerical sequence from 1 to 32. It does not provide narrative content or thematic information. The focus appears to be purely on listing numerical values.Electronics and Electrodynamics Notes 27-06-2024
Electronics and Electrodynamics Notes 27-06-2024Rajput AbdulWaheed Bhatti
╠²
The document lists various concepts and principles related to electromagnetics, circuits, and electrical components from multiple textbooks. It includes topics such as amplifiers, capacitance, impedance, and electromagnetic fundamentals, with references to various authors and editions. The extensive compilation highlights key electrical engineering topics and their respective sources.Vectors and Tensor Analysis 11-04-2024.doc
Vectors and Tensor Analysis 11-04-2024.docRajput AbdulWaheed Bhatti
╠²
The document contains two sections about two individuals, Robert C Wrede and Prasun Kumar Nayak. Robert C Wrede's section is brief and ends after line 8. The section about Prasun Kumar Nayak spans lines 9 through 37 and details a 373 page document he authored that ends on line 36. Both sections conclude with "The End".Lagrangian & Hamiltonian Mechanics 11-04-2024.doc
Lagrangian & Hamiltonian Mechanics 11-04-2024.docRajput AbdulWaheed Bhatti
╠²
This document appears to be a list of names, numbers, and short phrases. It includes the names John Tailor, Akhlaq Hussain, Dieter Strach, Wolfgang Notling, Vladimir Pletser, Fowler, and NIVALDO. Various page numbers are mentioned such as 291/413, 385 Daqaq, 371, 655 Pages, and 8888. Short phrases also appear including "Degree of Freedom", "Generalized Momenta", and "Hamiltonian". The document does not provide much context or narrative to summarize.Work Done By F.doc
Work Done By F.docRajput AbdulWaheed Bhatti
╠²
1. The document presents the solution to two mechanics problems (Problems 18 and 19) involving calculating displacement (d) and work (W) given position vectors.
2. For Problem 18, d is calculated as 2 units and W is calculated as 26 Nm.
3. For Problem 19, d is calculated as -1 units and W is calculated as 45 Nm.Visible Light Range.doc
Visible Light Range.docRajput AbdulWaheed Bhatti
╠²
The document summarizes the photon energies of visible light and FM radio frequencies.
(1) The range of photon energies in visible light is from 1.6533 eV to 3.2631 eV.
(2) The energy of a photon from a typical 100 MHz FM radio station broadcast is 4.136├Ś10-15 eV.TEMP of Blackbody.doc
TEMP of Blackbody.docRajput AbdulWaheed Bhatti
╠²
The document calculates the temperature of a blackbody with a peak spectrum of 700 nm. It uses Wien's displacement law, which relates the peak wavelength to the blackbody temperature. It shows the calculations for peak wavelengths in the visible light region at 700 nm, the microwave region at 3 cm, and the FM radio wave region at 3 m. In each case, it applies Wien's law and solves for the temperature in Kelvin. The temperatures calculated are 29,000 K for 700 nm visible light, 3,000 K for 3 cm microwaves, and 30,000 K for 3 m FM radio waves.Ratio Fb & Fg.doc
Ratio Fb & Fg.docRajput AbdulWaheed Bhatti
╠²
The document describes calculating the ratio of the magnetic force to the gravitational force on a proton moving vertically at the Earth's equator. It gives the proton's speed, the horizontal component of Earth's magnetic field, the proton's mass, and the gravitational acceleration. It then shows the calculations of the magnetic force using the magnetic field and speed, and the gravitational force using mass and gravitational acceleration. The ratio of magnetic to gravitational force is calculated to be approximately 16.R of Curvature.doc
R of Curvature.docRajput AbdulWaheed Bhatti
╠²
A beam of particles including protons, electrons, deuterons, and helium atoms all with a speed of 2.5 x 10^8 m/s passes through a magnetic field of 0.40 T perpendicular to their velocity. The radius of curvature of the path is calculated for each particle type using the formula that relates magnetic field, particle mass, charge, and velocity. The radius is found to be 6.52 x 10^-2 m for protons, 3.559 x 10^-1 m for electrons, 1.305 x 10^-1 m for deuterons, and 2.594 x 10^-1 m for helium atoms.Parallel Vectors.doc
Parallel Vectors.docRajput AbdulWaheed Bhatti
╠²
This document provides the solution to a math problem involving vectors. It shows that the vectors (1,3,3), (1,6,6), and (1,-6,-6) are parallel to each other and equal to zero when their dot products are calculated. This means the vectors are linearly dependent and span a line through the origin in three-dimensional space rather than filling out the full three-dimensional space.JB Marion 8.27.doc
JB Marion 8.27.docRajput AbdulWaheed Bhatti
╠²
The spacecraft orbits Earth with a speed of 10,160 m/s at a perigee of 6,680 km. Using the law of conservation of angular momentum, the speed of the spacecraft at its apogee of 42,200 km can be calculated. Substituting the given values into the angular momentum equation and solving for the apogee speed gives a value of 1,608.26 m/s.Gasiorovicz-3.doc
Gasiorovicz-3.docRajput AbdulWaheed Bhatti
╠²
Ultraviolet light with a wavelength of 350 nm was shone on a potassium surface, ejecting photoelectrons with a maximum kinetic energy of 1.6 eV. Using the formula that relates photon energy, work function, and kinetic energy, along with the given values, the work function of potassium was calculated to be 1.94 eV.Gasiorovicz 4.doc
Gasiorovicz 4.docRajput AbdulWaheed Bhatti
╠²
The document discusses using photoelectric emission data from aluminum to calculate Planck's constant and the work function of aluminum. It provides the maximum kinetic energies of photoelectrons emitted from aluminum when irradiated with 200nm and 258nm wavelength light. It then shows the calculations using Einstein's photoelectric equation to solve for Planck's constant and the work function. Planck's constant is calculated to be 6.626x10-34 Js and the work function of aluminum is calculated to be 3.9 eV.Fowles Cassiday 4.3.doc
Fowles Cassiday 4.3.docRajput AbdulWaheed Bhatti
╠²
The document discusses determining if a given force field is conservative. It provides two examples of force fields and shows that for a force field to be conservative, the curl of the force must be equal to zero at all points. Additionally, a conservative force field can be written as the gradient of a scalar potential function. For the two example force fields provided, the document calculates the curls and determines that they are equal to zero, showing that the forces are conservative and can be written as gradients of potential functions.Fowles Cassiday 4.2.doc
Fowles Cassiday 4.2.docRajput AbdulWaheed Bhatti
╠²
The document discusses conservative and non-conservative force fields. It states that a force field is conservative if its curl is zero everywhere, meaning the gradient of a scalar potential field can represent it. Option (a) represents a conservative field, while option (b) is not conservative because its curl is non-zero. Option (c) also represents a conservative field because its curl is equal to zero.Bright Star.doc
Bright Star.docRajput AbdulWaheed Bhatti
╠²
A bright star has an effective surface temperature of 20,000 K. Using Wien's law, the wavelength with maximum emission is 145 nm. This places it in the ultraviolet region of the electromagnetic spectrum, specifically in the far ultraviolet range between 100-200 nm.Black-Body R.doc
Black-Body R.docRajput AbdulWaheed Bhatti
╠²
1. The document finds the wavelength of blackbody radiation at temperatures of 3 K, 300 K, and 3000 K using Wien's displacement law.
2. At 3 K the wavelength is calculated to be 966 micrometers.
3. At 300 K the wavelength is calculated to be 9.66 millimeters.
4. At 3000 K the wavelength is calculated to be 966 nanometers.Area of a Triangle.doc
Area of a Triangle.docRajput AbdulWaheed Bhatti
╠²
This document provides the solution to a matrix problem. It involves a 3x3 matrix with entries (1,1,1), (2,3,4), (3,0,1). Several steps of algebraic manipulations are shown to solve for the values of i, j, k, and expressions for X in terms of i, j, k. The final values obtained are i=1, j=3, k=10.Area of a Triangle 22-25.doc
Area of a Triangle 22-25.docRajput AbdulWaheed Bhatti
╠²
The document provides solutions to 5 problems that calculate the area of triangles defined by 3D coordinate points. Each problem lists the 3 points that define the triangle, calculates the vectors between the points, finds the cross product of 2 vectors to obtain the area, and states the final area value.Stephen 4.30.doc
Stephen 4.30.docRajput AbdulWaheed Bhatti
╠²
The document discusses exciting a hydrogen atom from the n=2 state to a higher state using a 397 nm laser. It shows the calculations of the energy differences between states. The highest state the hydrogen atom can be excited to is n=7, as the energy of the 397 nm photon is enough to overcome the difference between the n=2 and n=7 states but not the n=7 and n=8 states.Stephen 4.25.doc
Stephen 4.25.docRajput AbdulWaheed Bhatti
╠²
(1) The binding energy of an electron in the ground state of deuterium is 13.6 eV.
(2) The binding energy of an electron in the ground state of He+ is 54.4 eV.
(3) The binding energy of an electron in the ground state of Be+++ is 217.6 eV.Stephen 4.24.doc
Stephen 4.24.docRajput AbdulWaheed Bhatti
╠²
The initial state of the hydrogen atom was n=1 and the final state was n=5. This is because the photon emitted had a wavelength of 95 nm, which corresponds to an energy difference of 0.55 eV between the initial and final states of the hydrogen atom. The only transition that satisfies this is from the first to the fifth energy level.PAUL 10.10.doc
PAUL 10.10.docRajput AbdulWaheed Bhatti
╠²
The document describes how to calculate the resistivity of copper using classical models. Given the mean free path and mean speed of electrons in copper at 300K, the resistivity is calculated to be 1.23x10-8 ╬®m. Using the same model and equations but with temperature of 100K, the resistivity is calculated to be 6.5x10-8 ╬®m. The classical model assumes temperature does not affect mean free path but does affect mean electron speed, following Maxwell-Boltzmann distribution, and this causes resistivity to increase with decreasing temperature.Paper 108 | ThoreauŌĆÖs Influence on Gandhi: The Evolution of Civil Disobedience
Paper 108 | ThoreauŌĆÖs Influence on Gandhi: The Evolution of Civil DisobedienceRajdeep Bavaliya
╠²
Dive into the powerful journey from ThoreauŌĆÖs 19thŌĆæcentury essay to GandhiŌĆÖs mass movement, and discover how one manŌĆÖs moral stand became the backbone of nonviolent resistance worldwide. Learn how conscience met strategy to spark revolutions, and why their legacy still inspires todayŌĆÖs social justice warriors. Uncover the evolution of civil disobedience. DonŌĆÖt forget to like, share, and follow for more deep dives into the ideas that changed the world.
M.A. Sem - 2 | Presentation
Presentation Season - 2
Paper - 108: The American Literature
Submitted Date: April 2, 2025
Paper Name: The American Literature
Topic: ThoreauŌĆÖs Influence on Gandhi: The Evolution of Civil Disobedience
[Please copy the link and paste it into any web browser to access the content.]
Video Link: https://youtu.be/HXeq6utg7iQ
For a more in-depth discussion of this presentation, please visit the full blog post at the following link: https://rajdeepbavaliya2.blogspot.com/2025/04/thoreau-s-influence-on-gandhi-the-evolution-of-civil-disobedience.html
Please visit this blog to explore additional presentations from this season:
Hashtags:
#CivilDisobedience #ThoreauToGandhi #NonviolentResistance #Satyagraha #Transcendentalism #SocialJustice #HistoryUncovered #GandhiLegacy #ThoreauInfluence #PeacefulProtest
Keyword Tags:
civil disobedience, Thoreau, Gandhi, Satyagraha, nonviolent protest, transcendentalism, moral resistance, Gandhi Thoreau connection, social change, political philosophyMore Related Content
More from Rajput AbdulWaheed Bhatti (20)
TEMP of Blackbody.doc
TEMP of Blackbody.docRajput AbdulWaheed Bhatti
╠²
The document calculates the temperature of a blackbody with a peak spectrum of 700 nm. It uses Wien's displacement law, which relates the peak wavelength to the blackbody temperature. It shows the calculations for peak wavelengths in the visible light region at 700 nm, the microwave region at 3 cm, and the FM radio wave region at 3 m. In each case, it applies Wien's law and solves for the temperature in Kelvin. The temperatures calculated are 29,000 K for 700 nm visible light, 3,000 K for 3 cm microwaves, and 30,000 K for 3 m FM radio waves.Ratio Fb & Fg.doc
Ratio Fb & Fg.docRajput AbdulWaheed Bhatti
╠²
The document describes calculating the ratio of the magnetic force to the gravitational force on a proton moving vertically at the Earth's equator. It gives the proton's speed, the horizontal component of Earth's magnetic field, the proton's mass, and the gravitational acceleration. It then shows the calculations of the magnetic force using the magnetic field and speed, and the gravitational force using mass and gravitational acceleration. The ratio of magnetic to gravitational force is calculated to be approximately 16.R of Curvature.doc
R of Curvature.docRajput AbdulWaheed Bhatti
╠²
A beam of particles including protons, electrons, deuterons, and helium atoms all with a speed of 2.5 x 10^8 m/s passes through a magnetic field of 0.40 T perpendicular to their velocity. The radius of curvature of the path is calculated for each particle type using the formula that relates magnetic field, particle mass, charge, and velocity. The radius is found to be 6.52 x 10^-2 m for protons, 3.559 x 10^-1 m for electrons, 1.305 x 10^-1 m for deuterons, and 2.594 x 10^-1 m for helium atoms.Parallel Vectors.doc
Parallel Vectors.docRajput AbdulWaheed Bhatti
╠²
This document provides the solution to a math problem involving vectors. It shows that the vectors (1,3,3), (1,6,6), and (1,-6,-6) are parallel to each other and equal to zero when their dot products are calculated. This means the vectors are linearly dependent and span a line through the origin in three-dimensional space rather than filling out the full three-dimensional space.JB Marion 8.27.doc
JB Marion 8.27.docRajput AbdulWaheed Bhatti
╠²
The spacecraft orbits Earth with a speed of 10,160 m/s at a perigee of 6,680 km. Using the law of conservation of angular momentum, the speed of the spacecraft at its apogee of 42,200 km can be calculated. Substituting the given values into the angular momentum equation and solving for the apogee speed gives a value of 1,608.26 m/s.Gasiorovicz-3.doc
Gasiorovicz-3.docRajput AbdulWaheed Bhatti
╠²
Ultraviolet light with a wavelength of 350 nm was shone on a potassium surface, ejecting photoelectrons with a maximum kinetic energy of 1.6 eV. Using the formula that relates photon energy, work function, and kinetic energy, along with the given values, the work function of potassium was calculated to be 1.94 eV.Gasiorovicz 4.doc
Gasiorovicz 4.docRajput AbdulWaheed Bhatti
╠²
The document discusses using photoelectric emission data from aluminum to calculate Planck's constant and the work function of aluminum. It provides the maximum kinetic energies of photoelectrons emitted from aluminum when irradiated with 200nm and 258nm wavelength light. It then shows the calculations using Einstein's photoelectric equation to solve for Planck's constant and the work function. Planck's constant is calculated to be 6.626x10-34 Js and the work function of aluminum is calculated to be 3.9 eV.Fowles Cassiday 4.3.doc
Fowles Cassiday 4.3.docRajput AbdulWaheed Bhatti
╠²
The document discusses determining if a given force field is conservative. It provides two examples of force fields and shows that for a force field to be conservative, the curl of the force must be equal to zero at all points. Additionally, a conservative force field can be written as the gradient of a scalar potential function. For the two example force fields provided, the document calculates the curls and determines that they are equal to zero, showing that the forces are conservative and can be written as gradients of potential functions.Fowles Cassiday 4.2.doc
Fowles Cassiday 4.2.docRajput AbdulWaheed Bhatti
╠²
The document discusses conservative and non-conservative force fields. It states that a force field is conservative if its curl is zero everywhere, meaning the gradient of a scalar potential field can represent it. Option (a) represents a conservative field, while option (b) is not conservative because its curl is non-zero. Option (c) also represents a conservative field because its curl is equal to zero.Bright Star.doc
Bright Star.docRajput AbdulWaheed Bhatti
╠²
A bright star has an effective surface temperature of 20,000 K. Using Wien's law, the wavelength with maximum emission is 145 nm. This places it in the ultraviolet region of the electromagnetic spectrum, specifically in the far ultraviolet range between 100-200 nm.Black-Body R.doc
Black-Body R.docRajput AbdulWaheed Bhatti
╠²
1. The document finds the wavelength of blackbody radiation at temperatures of 3 K, 300 K, and 3000 K using Wien's displacement law.
2. At 3 K the wavelength is calculated to be 966 micrometers.
3. At 300 K the wavelength is calculated to be 9.66 millimeters.
4. At 3000 K the wavelength is calculated to be 966 nanometers.Area of a Triangle.doc
Area of a Triangle.docRajput AbdulWaheed Bhatti
╠²
This document provides the solution to a matrix problem. It involves a 3x3 matrix with entries (1,1,1), (2,3,4), (3,0,1). Several steps of algebraic manipulations are shown to solve for the values of i, j, k, and expressions for X in terms of i, j, k. The final values obtained are i=1, j=3, k=10.Area of a Triangle 22-25.doc
Area of a Triangle 22-25.docRajput AbdulWaheed Bhatti
╠²
The document provides solutions to 5 problems that calculate the area of triangles defined by 3D coordinate points. Each problem lists the 3 points that define the triangle, calculates the vectors between the points, finds the cross product of 2 vectors to obtain the area, and states the final area value.Stephen 4.30.doc
Stephen 4.30.docRajput AbdulWaheed Bhatti
╠²
The document discusses exciting a hydrogen atom from the n=2 state to a higher state using a 397 nm laser. It shows the calculations of the energy differences between states. The highest state the hydrogen atom can be excited to is n=7, as the energy of the 397 nm photon is enough to overcome the difference between the n=2 and n=7 states but not the n=7 and n=8 states.Stephen 4.25.doc
Stephen 4.25.docRajput AbdulWaheed Bhatti
╠²
(1) The binding energy of an electron in the ground state of deuterium is 13.6 eV.
(2) The binding energy of an electron in the ground state of He+ is 54.4 eV.
(3) The binding energy of an electron in the ground state of Be+++ is 217.6 eV.Stephen 4.24.doc
Stephen 4.24.docRajput AbdulWaheed Bhatti
╠²
The initial state of the hydrogen atom was n=1 and the final state was n=5. This is because the photon emitted had a wavelength of 95 nm, which corresponds to an energy difference of 0.55 eV between the initial and final states of the hydrogen atom. The only transition that satisfies this is from the first to the fifth energy level.PAUL 10.10.doc
PAUL 10.10.docRajput AbdulWaheed Bhatti
╠²
The document describes how to calculate the resistivity of copper using classical models. Given the mean free path and mean speed of electrons in copper at 300K, the resistivity is calculated to be 1.23x10-8 ╬®m. Using the same model and equations but with temperature of 100K, the resistivity is calculated to be 6.5x10-8 ╬®m. The classical model assumes temperature does not affect mean free path but does affect mean electron speed, following Maxwell-Boltzmann distribution, and this causes resistivity to increase with decreasing temperature.Recently uploaded (20)
Paper 108 | ThoreauŌĆÖs Influence on Gandhi: The Evolution of Civil Disobedience
Paper 108 | ThoreauŌĆÖs Influence on Gandhi: The Evolution of Civil DisobedienceRajdeep Bavaliya
╠²
Dive into the powerful journey from ThoreauŌĆÖs 19thŌĆæcentury essay to GandhiŌĆÖs mass movement, and discover how one manŌĆÖs moral stand became the backbone of nonviolent resistance worldwide. Learn how conscience met strategy to spark revolutions, and why their legacy still inspires todayŌĆÖs social justice warriors. Uncover the evolution of civil disobedience. DonŌĆÖt forget to like, share, and follow for more deep dives into the ideas that changed the world.
M.A. Sem - 2 | Presentation
Presentation Season - 2
Paper - 108: The American Literature
Submitted Date: April 2, 2025
Paper Name: The American Literature
Topic: ThoreauŌĆÖs Influence on Gandhi: The Evolution of Civil Disobedience
[Please copy the link and paste it into any web browser to access the content.]
Video Link: https://youtu.be/HXeq6utg7iQ
For a more in-depth discussion of this presentation, please visit the full blog post at the following link: https://rajdeepbavaliya2.blogspot.com/2025/04/thoreau-s-influence-on-gandhi-the-evolution-of-civil-disobedience.html
Please visit this blog to explore additional presentations from this season:
Hashtags:
#CivilDisobedience #ThoreauToGandhi #NonviolentResistance #Satyagraha #Transcendentalism #SocialJustice #HistoryUncovered #GandhiLegacy #ThoreauInfluence #PeacefulProtest
Keyword Tags:
civil disobedience, Thoreau, Gandhi, Satyagraha, nonviolent protest, transcendentalism, moral resistance, Gandhi Thoreau connection, social change, political philosophyYSPH VMOC Special Report - Measles Outbreak Southwest US 6-14-2025.pptx
YSPH VMOC Special Report - Measles Outbreak Southwest US 6-14-2025.pptxYale School of Public Health - The Virtual Medical Operations Center (VMOC)
╠²
BLUF:
The Texas outbreak has slowed down, but sporadic cases continue to emerge in Kansas, Oklahoma, and New Mexico.
Elsewhere in the US, we continue to see signs of acceleration due to outbreaks outside the Southwest (North Dakota, Montana, and Colorado) and travel-related cases. Measles exposures due to travel are expected to pose a significant challenge throughout the summer.
The U.S. is on track to exceed its 30-year high for measles cases (1,274) within the next two weeks.
Here is the latest update:
CURRENT CASE COUNT: 919
ŌĆóTexas: 744 (+2) (55% of cases are in Gaines County).
ŌĆóNew Mexico: 81 (83% of cases are from Lea County).
ŌĆóOklahoma: 20 (+2)
ŌĆóKansas: 74 (+5) (38.89% of the cases are from Gray County).
HOSPITALIZATIONS: 104
ŌĆó Texas: 96 (+2) ŌĆō This accounts for 13% of all cases in Texas.
ŌĆó New Mexico: 7 ŌĆō This accounts for 9.47% of all cases in New Mexico.
ŌĆó Kansas: 3 ŌĆō This accounts for 5.08% of all cases in the state of Kansas.
DEATHS: 3
ŌĆóTexas: 2 ŌĆō This is 0.27% of all cases in Texas.
ŌĆóNew Mexico: 1 ŌĆō This is 1.23% of all cases in New Mexico.
US NATIONAL CASE COUNT: 1,197
INTERNATIONAL SPREAD
ŌĆóMexico: 2337 (+257), 5 fatalities
ŌĆÆChihuahua, Mexico: 2,179 (+239) cases, 4 fatalities, 7 currently hospitalized.
ŌĆóCanada: 3,207 (+208), 1 fatality
ŌĆÆOntario Outbreak, Canada: 2,115 (+74) cases, 158 hospitalizations, 1 fatality.
ŌĆÆAlberta, Canada: 879(+118) cases, 5 currently hospitalized.How to Manage Different Customer Addresses in Odoo 18 Accounting
How to Manage Different Customer Addresses in Odoo 18 AccountingCeline George
╠²
A business often have customers with multiple locations such as office, warehouse, home addresses and this feature allows us to associate with different addresses with each customer streamlining the process of creating sales order invoices and delivery orders.BINARY files CSV files JSON files with example.pptx
BINARY files CSV files JSON files with example.pptxRamakrishna Reddy Bijjam
╠²
BINARY FILES, CSV FILESGEOGRAPHY-Study Material [ Class 10th] .pdf
GEOGRAPHY-Study Material [ Class 10th] .pdfSHERAZ AHMAD LONE
╠²
"Geography Study Material for Class 10th" provides a comprehensive and easy-to-understand resource for key topics like Resources & Development, Water Resources, Agriculture, Minerals & Energy, Manufacturing Industries, and Lifelines of the National Economy. Designed as per the latest NCERT/JKBOSE syllabus, it includes notes, maps, diagrams, and MODEL question Paper to help students excel in exams. Whether revising for exams or strengthening conceptual clarity, this material ensures effective learning and high scores. Perfect for last-minute revisions and structured study sessions.Wax Moon, Richmond, VA. Terrence McPherson
Wax Moon, Richmond, VA. Terrence McPhersonTerrenceMcPherson1
╠²
Wax Moon is an independent record store keeping its foundational foothold in vinyl records by taking in collections and keeping the old 80s aesthetics alive with involvement in its community and participation with record distributors.Birnagar High School Platinum Jubilee Quiz.pptx
Birnagar High School Platinum Jubilee Quiz.pptxSourav Kr Podder
╠²
Birnagar High School Platinum Jubilee Celebration QuizPaper 107 | From Watchdog to Lapdog: IshiguroŌĆÖs Fiction and the Rise of ŌĆ£Godi...
Paper 107 | From Watchdog to Lapdog: IshiguroŌĆÖs Fiction and the Rise of ŌĆ£Godi...Rajdeep Bavaliya
╠²
Dive into a captivating analysis where Kazuo IshiguroŌĆÖs nuanced fiction meets the stark realities of postŌĆæ2014 Indian journalism. Uncover how ŌĆ£Godi MediaŌĆØ turned from watchdog to lapdog, echoing the moral compromises of IshiguroŌĆÖs protagonists. WeŌĆÖll draw parallels between restrained narrative silences and sensationalist headlinesŌĆöare our media heroes or traitors? DonŌĆÖt forget to follow for more deep dives!
M.A. Sem - 2 | Presentation
Presentation Season - 2
Paper - 107: The Twentieth Century Literature: From World War II to the End of the Century
Submitted Date: April 4, 2025
Paper Name: The Twentieth Century Literature: From World War II to the End of the Century
Topic: From Watchdog to Lapdog: IshiguroŌĆÖs Fiction and the Rise of ŌĆ£Godi MediaŌĆØ in Post-2014 Indian Journalism
[Please copy the link and paste it into any web browser to access the content.]
Video Link: https://youtu.be/kIEqwzhHJ54
For a more in-depth discussion of this presentation, please visit the full blog post at the following link: https://rajdeepbavaliya2.blogspot.com/2025/04/from-watchdog-to-lapdog-ishiguro-s-fiction-and-the-rise-of-godi-media-in-post-2014-indian-journalism.html
Please visit this blog to explore additional presentations from this season:
Hashtags:
#GodiMedia #Ishiguro #MediaEthics #WatchdogVsLapdog #IndianJournalism #PressFreedom #LiteraryCritique #AnArtistOfTheFloatingWorld #MediaCapture #KazuoIshiguro
Keyword Tags:
Godi Media, Ishiguro fiction, post-2014 Indian journalism, media capture, Kazuo Ishiguro analysis, watchdog to lapdog, press freedom India, media ethics, literature and media, An Artist of the Floating WorldQ1_ENGLISH_PPT_WEEK 1 power point grade 3 Quarter 1 week 1
Q1_ENGLISH_PPT_WEEK 1 power point grade 3 Quarter 1 week 1jutaydeonne
╠²
Grade 3 Quarter 1 Week 1 English part 2ROLE PLAY: FIRST AID -CPR & RECOVERY POSITION.pptx
ROLE PLAY: FIRST AID -CPR & RECOVERY POSITION.pptxBelicia R.S
╠²
Role play : First Aid- CPR, Recovery position and Hand hygiene.
Scene 1: Three friends are shopping in a mall
Scene 2: One of the friend becomes victim to electric shock.
Scene 3: Arrival of a first aider
Steps:
Safety First
Evaluate the victimŌĆśs condition
Call for help
Perform CPR- Secure an open airway, Chest compression, Recuse breaths.
Put the victim in Recovery position if unconscious and breathing normally.
Sustainable Innovation with Immersive Learning
Sustainable Innovation with Immersive LearningLeonel Morgado
╠²
Prof. Leonel and Prof. Dennis approached educational uses, practices, and strategies of using immersion as a lens to interpret, design, and planning educational activities in a sustainable way. Rather than one-off gimmicks, the intent is to enable instructors (and institutions) to be able to include them in their regular activities, including the ability to evaluate and redesign them.
Immersion as a phenomenon enables interpreting pedagogical activities in a learning-agnostic way: you take a stance on the learning theory to follow, and leverage immersion to envision and guide your practice.Paper 109 | Archetypal Journeys in ŌĆśInterstellarŌĆÖ: Exploring Universal Themes...
Paper 109 | Archetypal Journeys in ŌĆśInterstellarŌĆÖ: Exploring Universal Themes...Rajdeep Bavaliya
╠²
Get ready to embark on a cosmic quest as we unpack the archetypal power behind Christopher NolanŌĆÖs ŌĆśInterstellar.ŌĆÖ Discover how heroŌĆÖs journey tropes, mythic symbols like wormholes and tesseracts, and themes of love, sacrifice, and environmental urgency shape this epic odyssey. Whether youŌĆÖre a film theory buff or a casual viewer, youŌĆÖll learn why CooperŌĆÖs journey resonates with timeless mythsŌĆöand what it means for our own future. Smash that like button, and follow for more deep dives into cinemaŌĆÖs greatest stories!
M.A. Sem - 2 | Presentation
Presentation Season - 2
Paper - 109: Literary Theory & Criticism and Indian Aesthetics
Submitted Date: April 5, 2025
Paper Name: Literary Theory & Criticism and Indian Aesthetics
Topic: Archetypal Journeys in ŌĆśInterstellarŌĆÖ: Exploring Universal Themes in NolanŌĆÖs Cosmic Odyssey
[Please copy the link and paste it into any web browser to access the content.]
Video Link: https://youtu.be/vHLaLZPHumk
For a more in-depth discussion of this presentation, please visit the full blog post at the following link: https://rajdeepbavaliya2.blogspot.com/2025/04/archetypal-journeys-in-interstellar-exploring-universal-themes-in-nolan-s-cosmic-odyssey.html
Please visit this blog to explore additional presentations from this season:
Hashtags:
#ChristopherNolan #Interstellar #NolanFilms #HeroJourney #CosmicOdyssey #FilmTheory #ArchetypalCriticism #SciFiCinema #TimeDilation #EnvironmentalCinema #MythicStorytelling
Keyword Tags:
Interstellar analysis, Christopher Nolan archetypes, heroŌĆÖs journey explained, wormhole symbolism, tesseract meaning, myth in sci-fi, cinematic archetypes, environmental themes film, love across time, Nolan film breakdownChalukyas of Gujrat, Solanki Dynasty NEP.pptx
Chalukyas of Gujrat, Solanki Dynasty NEP.pptxDr. Ravi Shankar Arya Mahila P. G. College, Banaras Hindu University, Varanasi, India.
╠²
This presentation has been made keeping in mind the students of undergraduate and postgraduate level. In this slide try to present the brief history of Chaulukyas of Gujrat up to Kumarpala To keep the facts in a natural form and to display the material in more detail, the help of various books, websites and online medium has been taken. Whatever medium the material or facts have been taken from, an attempt has been made by the presenter to give their reference at the end.
Chaulukya or Solanki was one of the Rajputs born from Agnikul. In the Vadnagar inscription, the origin of this dynasty is told from Brahma's Chauluk or Kamandalu. They ruled in Gujarat from the latter half of the tenth century to the beginning of the thirteenth century. Their capital was in Anahilwad. It is not certain whether it had any relation with the Chalukya dynasty of the south or not. It is worth mentioning that the name of the dynasty of the south was 'Chaluky' while the dynasty of Gujarat has been called 'Chaulukya'. The rulers of this dynasty were the supporters and patrons of Jainism.Revista digital preescolar en transformaci├│n
Revista digital preescolar en transformaci├│nguerragallardo26
╠²
EVOLUCI├ōN DEL CONTENIDO DE LA EVALUACI├ōN DE LOS RECURSOS Y DE LA FORMACI├ōN DE LOS DOCENTESLDMMIA Practitioner Level Orientation Updates
LDMMIA Practitioner Level Orientation UpdatesLDM & Mia eStudios
╠²
See our 2 Starter PDFs within a Compressed, Zip Drive. Within Shop. Videos will be available Before the Weekend of 6/14th. For the US, Happy Fathers Day Weekend. (Our readers/teams are global.) Also, our content remains timeless for Future Grad Students seeking updates.
After about a Year or 10, I retire older content. Literally up to under 10 yrs. We will be 19 yrs old this Aug for Love and Divinity in Motion (LDM). How old are we? So funny. Our oldest profile is X, formerly Twitter. From our old Apple Podcast Years.
https://ldm-mia.creator-spring.com
Session/Lesson 1 -Intro
REIKI- YOGA ŌĆ£ORIENTATIONŌĆØ
It helps to understand the text behind anything. This improves our performance and confidence.
Your training will be mixed media. Includes Rehab Intro and Meditation vods, all sold separately.
Editing our Vods & New Shop. Retail under $30 per item.
*Store Fees will apply. *Digital Should be low cost.
Thank you for attending our free workshops. Those can be used with any Reiki Yoga training package. Traditional Reiki does host rules and ethics. Its silent and within the JP Culture/Area/Training/Word of Mouth. It allows remote healing but thereŌĆÖs limits for practitioners and masters. We are not allowed to share certain secrets/tools. Some content is designed only for ŌĆ£MastersŌĆØ...
Next Upload will be our Video package for Session 1. Prices will be affordable as possible. Thx for becoming a "Practitioner Level" Student.
Updates so far, are every week for spring. Summer should be a similar schedule. Thx for visitings, attending, and following LDMMIA.
Social Media:
https://x.com/OnlineDrLeZ
and
https://www.instagram.com/chelleofsl/
ABCs of Bookkeeping for Nonprofits TechSoup.pdf
ABCs of Bookkeeping for Nonprofits TechSoup.pdfTechSoup
╠²
Accounting can be hard enough if you havenŌĆÖt studied it in school. Nonprofit accounting is actually very different and more challenging still.
Need help? Join Nonprofit CPA and QuickBooks expert Gregg Bossen in this first-time webinar and learn the ABCs of keeping books for a nonprofit organization.
Key takeaways
* What accounting is and how it works
* How to read a financial statement
* What financial statements should be given to the board each month
* What three things nonprofits are required to track
What features to use in QuickBooks to track programs and grantsThis is why students from these 44 institutions have not received National Se...
This is why students from these 44 institutions have not received National Se...Kweku Zurek
╠²
This is why students from these 44 institutions have not received National Service PIN codes (LIST)Pests of Maize: An comprehensive overview.pptx
Pests of Maize: An comprehensive overview.pptxArshad Shaikh
╠²
Maize is susceptible to various pests that can significantly impact yields. Key pests include the fall armyworm, stem borers, cob earworms, shoot fly. These pests can cause extensive damage, from leaf feeding and stalk tunneling to grain destruction. Effective management strategies, such as integrated pest management (IPM), resistant varieties, biological control, and judicious use of chemicals, are essential to mitigate losses and ensure sustainable maize production.YSPH VMOC Special Report - Measles Outbreak Southwest US 6-14-2025.pptx
YSPH VMOC Special Report - Measles Outbreak Southwest US 6-14-2025.pptxYale School of Public Health - The Virtual Medical Operations Center (VMOC)
╠²
Paper 107 | From Watchdog to Lapdog: IshiguroŌĆÖs Fiction and the Rise of ŌĆ£Godi...
Paper 107 | From Watchdog to Lapdog: IshiguroŌĆÖs Fiction and the Rise of ŌĆ£Godi...Rajdeep Bavaliya
╠²
Paper 109 | Archetypal Journeys in ŌĆśInterstellarŌĆÖ: Exploring Universal Themes...
Paper 109 | Archetypal Journeys in ŌĆśInterstellarŌĆÖ: Exploring Universal Themes...Rajdeep Bavaliya
╠²
Chalukyas of Gujrat, Solanki Dynasty NEP.pptx
Chalukyas of Gujrat, Solanki Dynasty NEP.pptxDr. Ravi Shankar Arya Mahila P. G. College, Banaras Hindu University, Varanasi, India.
╠²
Ad
Stephen 4.31.doc
- 1. 1 ’Ć© ’Ć® ’Ć© ’Ć® ’Ć© ’Ć® 2 A muonic atom consists of a muon mass 106 / and charge - in place of an electron. For the muon in a hydrogen atom, what is a the smallest radius and b the binding energy of the muon in the gr m MeV c q e ’ĆĮ ’ĆĮ ound state? Solution
- 2. 2
- 3. 3 2 2 Let be the reduced mass, 106 be the mass of muon, 938.27 be the mass of the proton, then MeV m c MeV M c ’üŁ ’ĆĮ ’ĆĮ
- 4. 4 2 2 2 2 2 2 2 2 = 106 938.27 106 938.27 99456.62( ) 1044.27 95.24 mM m M MeV MeV X c c MeV MeV c c MeV c MeV c MeV c ’üŁ ’Ć½ ’ĆĮ ’Ć½ ’ĆĮ ’ĆĮ
- 5. 5 (a) 2 0 0 2 2 12 2 15 2 2 19 2 2 2 2 42 2 38 6 2 2 Using 8.854 10 . (4.136 10 . ) 3.14 95.24 (1.602 10 ) . . 151.46 10 . 767.49 10 10 h a e C X X N m X eV s MeV X X c X C C eV s X N m eV X X XC c ’üź ’ü░’üŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆĮ ’ĆĮ ’ĆĮ
- 6. 6 10 2 2 2 10 2 16 2 2 2 6 6 19 13 13 13 0.197 10 . . . 0.197 10 9 10 . . . 1.78 10 1.78 10 1.602 10 2.85 10 2.85 10 . 2.85 10 X eV s c N m X X X m eV s s N m X eV N X X X J N X J N X N m N X m ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆĮ ’ĆĮ ’ĆĮ ’ĆĮ ’ĆĮ ’ĆĮ ’ĆĮ
- 7. 7 (b) 4 2 2 0 2 2 15 2 Using 2 (4 ) 95.24 (1.440 . ) 2 (4.136 10 . ) 1 4 3.14 3.14 me E MeV X c eV nm X X eV s X X X ’ü░’üź ’ĆŁ ’ĆĮ ’ĆĮ
- 8. 8 6 2 2 18 2 30 2 2 2 12 2 30 2 18 2 2 2 18 2 2 16 2 2 2 95.24 10 2.07 10 0.87 10 . . 197.15 10 0.87 10 226.61 10 . . 226.61 10 . 9 10 . 25.18 10 2518 eV X X c XeV X m X eV s eV m X c X s X eV m c s X eV m m X s s X eV eV ’ĆŁ ’ĆŁ ’ĆŁ ’ĆŁ ’ĆĮ ’ĆĮ ’ĆĮ ’ĆĮ ’ĆĮ ’ĆĮ
