UNTITLED.ppt
- 1. Implementing a Holistic Approach to your Quality Management System Steven R. Cagle V.P. of Marketing & Product Development Sparta Systems, Inc.
- 2. 2 Agenda ïŪ Session Objectives ïŪ Quality Management System Overview ïŪ Traditional Challenges ïŪ Re-defining CAPA ïŪ Implementing a Quality Management Software Solution ïŪ Conclusion ïŪ Q&A
- 3. 3 Session Objective Discuss critical components of an effective Quality Management System (QMS), challenges with current systems, and solutions to overcome these challenges by implementing a holistic Quality Management Software solution.
- 5. 5 Defining CAPA â ISO 13485:2003 ïŪ 8.5.2 Corrective Action â Corrective actions shall be appropriate to the effects of the nonconformities encountered. A documented procedure shall be established to define requirements for a) reviewing nonconformities (including customer complaints) b) determining the cause of nonconformities c) evaluating the need for action to ensure that nonconformities to not recur d) determining and implementing action needed, including, if appropriate, updating documentation e) recording of the results of any investigation and of action taken, and f) reviewing the corrective action taken and its effectiveness
- 6. 6 Defining CAPA â ISO 13485:2003 ïŪ 8.5.3 Preventive action â The organization shall determine action to eliminate the causes of potential nonconformities in order to prevent their occurrence. Preventive actions shall be appropriate to the effects of the potential problems. ïŪ It is also determine potential nonconformities and their causes a) evaluating the need for action to prevent occurrence of nonconformities b) determining and implementing action needed c) recording of the results of any investigations and of action taken, and d) reviewing preventive action taken and its effectiveness
- 7. 7 Quality Regulation 21 CFR 820.100 ïŪ U.S. Food and Drug Administrationâs regulation governing medical device manufacturers quality systems: (a) Each manufacturer shall establish and maintain procedures for implementing corrective and preventive action: (1) analyzing processes, work operations, concessions, quality audit reports, quality records, service records, complaints, returned products, and other sources of quality data to identify existing and potential causes of nonconforming product, or other quality problems (2) investigating the cause of nonconformities relating to product, processes, and the quality system (3) identifying the actions needed to correct and prevent recurrence of nonconforming product and other quality problems (4) verifying or validating the corrective and preventive action (5) Implementing and recording changes in methods and procedures needed to correct and prevent identified quality problems (6) Ensuring that information related to quality problems or nonconforming product is disseminated to those directly responsible for assuring the quality of such product or the prevention of such problems; and (7) Submitting relevant information on identified quality problems, as well as corrective and preventive actions, for management review
- 8. 8 Quality Regulation 21 CFR 211.22 ïŪ Very similar is the U.S. FDAâs regulation for pharmaceutical manufacturers 21 CFR Part 211.22 (Quality Control Unit)âĶ responsibilities of a quality control unit...to assure that no errors have occurred or, if errors have occurred, that they have been fully investigated. The quality control unit shall have the responsibility for approving or rejecting all procedures or specifications impacting on the identity, strength, quality, an purity of the drug product ïŪ âĶand in Part 211.92 (Production Record Review) Any unexplained discrepancyâĶor the failure of a batch or any of its components to meet any of its specifications shall be thoroughly investigatedâĶThe investigation shall extend to other batches of the same drug product and other drug products that may have been associated with the specific failure or discrepancy. A written record of the investigation shall be made and shall include the conclusions and follow-up.
- 9. 9 Quotes from Current FDA WarningLetters ïŪ Each manufacturer shall establish procedures for quality audits and conduct such audits to assure that the quality system is in compliance with the established quality system requirements and to determine the effectiveness of the quality system . Quality audits shall be conducted by individuals who do not have direct responsibility for the matters and shall be taken when necessary. A report of the results of each quality audit, and reaudit(s) where taken, shall be made and such reports shall be reviewed by management having responsibility for the matters audited. The dates and results of quality audits and reaudits shall be documented as required by 21 CFR 820.22 . internal quality audits conducted by your firm failed to verify that the quality system was effective in fulfilling quality system objectives (FDA 483, Item #2).
- 10. 10 Quotes from Current FDA Warning Letters ïŪ Your firm fails to implement and maintain corrective and preventive action (CAPA) procedures that include requirements for analyzing processes, work operations, concessions, quality audit reports, quality records, service records, complaints, returned product, and other sources of quality data to identify existing and potential causes of nonconforming product, or other quality problems as required by 21 CFR 820.1 00(a)(1). ïŪ Your firm fails to establish and implement corrective and preventive action (CAPA) procedures that include requirements for identifying the action(s) needed to correct and prevent recurrence of non-conforming product and other quality problems as required by 21 CFR 820.100(a)(3) ïŪ All activities required by 21 CFR 820.100 must be verified or validated to ensure that such action is effective and does not adversely affect finished devices, and the results of these activities shall be documented as required by 21 CFR 820.100(a)(4) and (b). Your firm's CAPA procedures fail to document how analysis is done and fails to require verification/validation that CAPA does not adversely affect finished devices (FDA 483, Item #8).
- 11. 11 Commission Directive 2003/94/EC Preamble Having regard to the Treaty establishing the European Community, All manufacturers should operate an effective quality management system of their manufacturing operations, which requires the implementation of a pharmaceutical quality assurance system. Article 13 - Complaints Any complaint concerning a defect shall be recorded and investigated by the manufacturerâĶ Article 14 - Inspections The manufacturer shall conduct repeated self-inspectionsâĶin order to monitor the implementation and respect of good manufacturing practice and to propose any necessary corrective measures. Records shall be maintained of such self-inspections and any corrective action subsequently taken.
- 12. 12 More than just corrective actionsâĶ CAPA is much more than just âcorrective actionsâ and âpreventive actionsâ. Any opportunity to improve quality in your organization is a CAPA!
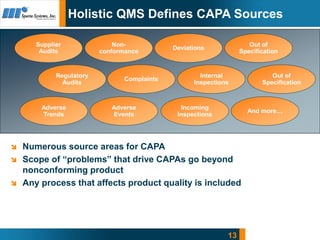
- 13. 13 Holistic QMS Defines CAPA Sources Complaints Internal Inspections Supplier Audits Regulatory Audits Non- conformance Deviations Out of Specification Out of Specification Adverse Trends Adverse Events Incoming Inspections And moreâĶ ïŪ Numerous source areas for CAPA ïŪ Scope of âproblemsâ that drive CAPAs go beyond nonconforming product ïŪ Any process that affects product quality is included
- 14. 14 CAPA Process â best practices Verify Effectiveness Implement Actions Review & Approve Plan Investigate, Root Cause, Action Plan Identify & Triage ïŪ Regardless of where the problem originates, or what type it is, it must follow a process ïIdentify problem ïAssess impact ïQuality / Regulatory / Management Notification ïInvestigation Process? ïComplete Investigation ïDetermine Root Cause ïProposed Corrective / Preventive Actions ïPlan effectiveness ïAssess changes ïEnsure no impact to product quality ïConsensus from SMEs ïApproval ïImplement Actions ïVerify completed ïInform stakeholders ïMeasure to ensure problem has been resolved ïMonitor to ensure it is not re-occurring Change Control Metrics and Reporting
- 16. 16 Typical QMS Challenges ïŪ Challenges in Problem Identification Missing view of the big picture Lack of ownership and accountability Inability to link related problems Insufficient tools for trending and analysis ïŪ Challenges in Investigation Quality of investigations is poor ï§ Missing & incomplete information ï§ Inability to easily review similar past investigations Inconsistent investigation process & Root Cause Not determining root cause Past due investigations, not being closed, get lost Problem Identification Identify & Investigat e Root Cause Create Action Plan ïŪ Challenges in Planning Vague root cause analysis Confusion over what is âcorrectiveâ and what is âpreventiveâ action Inability to relate corrective actions to source problems Lack of integration to Change Control System
- 17. 17 Typical QMS Challenges (cont.) Verify Effectiven ess Implement Actions ïŪ Challenges in Implementation No way to track issues through workflow Lack of visibility to open items Lack of visibility to related items Changes to plan mid-stream Compliance risk ïŪ Challenges in Effectiveness Easy to âforgetâ to measure effectiveness Difficult to gather necessary metrics No means to generate metrics Inability to measure effectiveness does not give us any assurance if we are addressing the root cause of the problems Review & Approve Plan ïŪ Challenges in Review & Approval Not sure who needs to approve Approvals in serial, not parallel Approval process takes long time Lack of key stakeholder input
- 18. 18 Solution Holistic Approach to Quality Management ïŪ Globalize (harmonize) around a common philosophy and approach to CAPA and source Events ïŪ Obtain full compliance with cGxPs, as well as regulatory & customer expectations ïŪ Use quality metrics as a basis for continuous improvements Trending â Problem Analysis Thorough Investigations and Root Cause Analysis Ensuring CAPA effectiveness Bring attention to risk areas to prevent problems Implement a centralized Quality Management System: ïŪ Manages all inputs and outputs as well as the actual actions ïŪ Scalable to be deployed on a global basis ïŪ Functionality / Flexibility to meet business requirements
- 19. Re-defining CAPA
- 20. 20 Re-defining CAPA ïŪ Definitions Standardize definitions across the organization Terms like â âdeviationâ, âeventâ, ânonconformanceâ, correctionâ, âcorrective actionâ, âpreventive actionâ, âdiscrepancyâ must be consistent for each operating unit The same term should have the same meaning everywhere, and drive the same process CAPA sources include: Complaints, Audits Observations, Trends can feed CAPA ïŪ Determine, scope identification & impact of new system Where does the process need to change? Who will the system affect? What existing policies may change? Understand the difference between the âwhatâ and the âwhoâ
- 21. Define the Inputs & Process
- 22. 22 Record the Event ïŪ Capture all related data of any event regardless of the type Source Date & Time of Event Type Description Department ïŪ For issues surrounding Events, utilize a quality evaluation: Quality Event only Quality Event + CAPA Quality Event + Investigation + CAPA + Change Control ïŪ Log observations / trends to implement pro-active changes
- 23. 23 Perform Assessment & Investigation ïŪ Assign Investigator Use âPushâ or âPullâ concept Assess impact, consider decision tree approach ïŪ Create Investigation Plan Use Parent-Child concepts â track each investigation âtaskâ Use Investigation Templates ïŪ Track & Complete Investigations Use workflow, due dates and reminders Escalation of past due investigations Search & Reporting User Dashboards ïŪ Analyze Root Cause Structure Root cause Analysis Tree Use Root Cause to Drive CAPA process
- 24. 24 CAPA Plan & Approval ïŪ Review currently in progress CAPAs ïŪ Create CAPAs and link to root cause If multiple CAPAs identify which ones resolve which root cause? Which actions must be closed to close the deviation? ïŪ Create an Effectiveness Plan at this time ïŪ Determine Approvers Use pre-set approver functions if possible ïŪ Route Investigation & CAPA plan for approval Email alerts, reminders Dashboards ïŪ Obtain Approval Ability to reject to various previous workflow states
- 25. 25 Implementing CAPA & Effectiveness ïŪ Each CAPA record should have its own record and workflow ïŪ Use Parent-child relationships to break up the process into âsmaller bitesâ Action Item Tracking ïŪ Track completion and verification of each CAPA Use workflow, due dates and reminders Escalation of past due investigations Search & Reporting User Dashboards ïŪ Measure effectiveness according to the plan -> evidence that root cause has been eliminated
- 27. 27 High Level Requirements Centralized database Handles all process areas - modular Workflow driven Proactive user notification and escalation Action items management Querying & Reporting Elaborate security by user-group Defining the Requirements Management reports Performance Metrics & Trending Part 11 Compliance
- 28. 28 Management of all Data ïŪ Modular approach to handling all source areas but maintains individual requirement Multiple âRecord Typesâ to handle all process areas Ability to create user defined fields Configurable data entry forms Validation and business rules ïŪ Integration to external systems E.g., Create deviations automatically from ERP E.g., Create OOS Investigation from LIMS Master data (customer, product/item, etc.) External Systems Data Management
- 29. 29 Workflow Management ïŪ Configurable workflow Automate review and approval process based on meta data Business-rule based workflows Parallel Approvals Process changing activity E-mail notifications ïŪ Integrated source areas process to Corrective Action process Parent â child relationships Cross referencing Workflow External Systems Data Management
- 30. 30 Escalations and Business Rules ïŪ Business rules enforcement Date Due, Milestone Dates Automatically assigning investigators, reviewers, approvers Automatically scheduling tasks based on type of Record ïŪ Escalation âRemindersâ of tasks reaching expected completion date Escalation of CAPA past due Workflow External Systems Data Management Business Rules & Escalation
- 31. 31 Query, Reporting, Trending Workflow External Systems Data Management Business Rules & Escalation Search, Report, Trend ïŪ Querying Ability to query on all fields Full text / search engine functionality Ability to save searches ïŪ Reporting Customizable report format On screen view, print, email, save Status reporting ïŪ Trending Across all sites Across all source areas Root cause analysis Identify occurrence rate decrease / increase Ability to detect trends automatically
- 32. 32 Compliance with Part 11 ïŪ Does the system conform with your firmâs Part 11 requirements? ïŪ Has the software âpassedâ the test, I.e., has it gone through FDA audits at another firm? ïŪ Can it be validated? ïŪ How confident are you in the above assessment? ïŪ Full audit trail ïŪ Reporting features ïŪ Configurable security groups ïŪ Complete record of created and modified data ïŪ Enforced workflow sequencing ïŪ Password composition rules ïŪ Electronic Signatures made up of two unique components ïŪ Password aging/expiration ïŪ Cannot re-use previous password ïŪ Account Locking and Admin Notification after failed log-in Attempts ïŪ Session time-out ïŪ Requirement of âreasonâ for data modification ïŪ Administrator / Configuration Audit Trail
- 34. 34 Quality, Operations, IT Structured Project Organization Customer â System Administrator Implementation Consultant Project Management Customer Executive Sponsor Sr. Mgmt. Support Business & IT Project Mgr. Vendor Project Manager Project Team âĒ Change Management âĒ Validation Partners âĒ Operations management Vendor SMEs QA & Customer Satisfaction Technical Support LAN/WAN DBA Servers Customer IT Sponsor Medtronic Leads: Medtronic Leads: Customer. Owner Team âĒ CAPA âĒ Complaints âĒ Audits âĒ RMAs âĒ Others Steering Committee âĒ Internal Computer Systems Validation âĒ Vendor âĒ 3rd Party (recommended) Validation Team Vendor Administrative Resources
- 35. 35 Rapid ROI - Phased Approach Critical Systems Prioritization Phase-1: FIRST QMS Configure, Prototype, Train Validation Phase-2: Additional QS Implementation, Configure, Prototype, Train DATA Migration & Systems Integration Reduced Validation via Migration tool Fully integrated CAPA Initial ROI / Business and compliance Benefits Roll out â Production Additional QS Yes No Prioritized QMS List and implementation Project Plan Go Live!
- 36. 36 âĒ Project Team Workshop-2 âĒ Finalize Configuration Design âĒ Reports Customization âĒ Train on Validation Templates âĒ Develop Validation Protocols âĒ Complete setup of integration tools âĒ Develop User Requirement Definition âĒ Functional specifications âĒ Initial validation activities âĒ Product Orientation âĒ Detailed âAs Isâ of current processes, organization and systems âĒ Visioning session for âto-beâ concepts âĒ System Configuration Design âĒ Installation of Development Environment âĒ Configure Prototype 1 âĒ Project Team Workshop-1 âĒ Configure Prototype 2 Requirements / Configuration Design Validation / SOPs / Training Add additional Projects / enhance projects Prototyping / Finalize Configuration / Documentation âĒ Support application âĒ Enhance configuration âĒ Add additional project areas to support additional needs âĒ PQ âĒ Leverage Migrator for reduced validation âĒ Roll out phase Project Phases Post Release âĒ Install Production âĒ Execute and approve IQ âĒ Execute and approve OQ âĒ Execute and approve PQ âĒ Complete validation protocol âĒ Create Training Materials âĒ Revise SOPs âĒ Train Super Users âĒ Train Users âĒ Train Help Desk âĒ Roll out phase 2-3 weeks 4-8 weeks 6-8 weeks 4â6 weeks per project Go Live! Implementation Schedule
- 37. Conclusion
- 38. 38 Conclusion (cont.) ïŪ QMS encompasses the overall process related to events / observations from multiple sources, as well as their investigations, and resolutions ïŪ A âbest practicesâ QMS system requires a single, scalable system, which uses a holistic approach ïŪ Implementing a global QMS system may require your organization to change how it defines CAPA as well as it how it conducts the CAPA process ïŪ Implementing a system can be successfully accomplished by using established software, a harmonized approach, and an organized project structure
- 39. 39 Q & A










































































![Traktor Pro Crack + License Key Free Download [2025]](https://cdn.slidesharecdn.com/ss_thumbnails/generalsciencenotes-250303184328-484ccf79-thumbnail.jpg?width=560&fit=bounds)










