Atomic Absorption Spectrometry (AAS)
- 1. Atomic Absorption Spectrometry (AAS) Yoddy A. Nuhgraha 23711301 Program Studi Ilmu & Teknik Material Fakultas Teknik Mesin & Dirgantara Institut Teknologi Bandung 2012
- 2. Outline 1. Pendahuluan 2. Kegunaan 3. Limitasi 4. Prinsip Dasar 5. Instrumentasi 6. Sampel 7. Kalibrasi 8. Gangguan 9. Lokasi 10. Metoda Lain 11. Pustaka Courtesy http://www.civil.iitm.ac.in Courtesy http://www.civil.iitm.ac.in 11/8/2012 2
- 3. WARNING ŌĆó These slides have not been extensively proof- read, and therefore may contain errors. ŌĆó While I have tried to cite all references, I may have missed someŌĆō these slides were prepared for an informal lecture and not for publication. ŌĆó If you note a mistake or a missing citation, please let me know and I will correct it. ŌĆó I hope to add commentary in the notes section of these slides, offering additional details. However, these notes are incomplete so far. 11/8/2012 http://prism.mit.edu/xray 3
- 4. 1. Pendahuluan ŌĆó AAS adalah prosedur analisa spektro untuk menentukan unsur kimia kuantitatif menggunakan penyerapan radiasi optik (cahaya) oleh atom bebas dalam bentuk gas ŌĆó Ilmuwan pengembang AAS ’ā╝Robert W. Bunsen & Gustav R. Kirchhoff (Jerman 1850an) ’ā╝Sir Alan Walsh (Australia 1950an) 11/8/2012 4
- 5. 2. Kegunaan ŌĆó Medis: kandungan logam pada darah atau urine. ŌĆó Lingkungan: kandungan logam dan unsur lain pada air, tanah, tanaman, kompos. ŌĆó Obat-obatan: katalis yang digunakan; logam berbahaya (Cd, Pb, etc). ŌĆó Industri: logam pada material mentah, logam mulia. ŌĆó Forensik: serbuk mesiu, keracunan 11/8/2012 5
- 6. 3. Limitasi ŌĆó Rentang pendeteksian mulai dari sub-parts per billion (ppb) sampai dengan parts per million (ppm) ŌĆó Tidak dapat menganalisa secara langsung untuk gas mulia, halogen, sulfur, karbon, atau nitrogen. ŌĆó Sensitifitas yang kurang untuk oksida refraktori atau unsur pembentuk karbida ŌĆó Secara mendasar merupakan teknik unsur tunggal 11/8/2012 6
- 7. Limitasi Elements detectable by atomic absorption are highlighted in pink in this periodic table. http://www.webapps.cee.vt.edu/ewr/environmental/teach/smprimer/aa/aa.html 11/8/2012 7
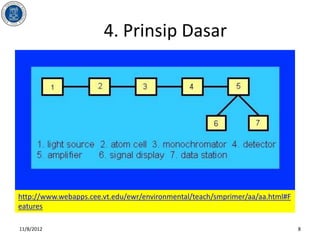
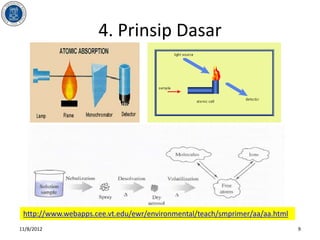
- 9. 4. Prinsip Dasar http://www.webapps.cee.vt.edu/ewr/environmental/teach/smprimer/aa/aa.html 11/8/2012 9
- 10. 5. Instrumentasi http://www.files.chem.vt.edu/chem-ed/spec/atomic/aa.html 11/8/2012 10
- 11. Flame AAS http://www.files.chem.vt.edu/chem-ed/spec/atomic/aa.html 11/8/2012 11
- 12. Graphite Furnace AAS http://www.files.chem.vt.edu/chem-ed/spec/atomic/aa.html 11/8/2012 12
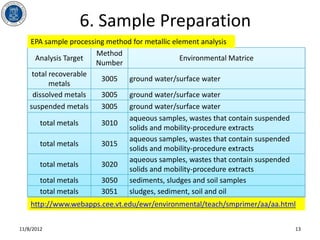
- 13. 6. Sample Preparation EPA sample processing method for metallic element analysis Method Analysis Target Environmental Matrice Number total recoverable 3005 ground water/surface water metals dissolved metals 3005 ground water/surface water suspended metals 3005 ground water/surface water aqueous samples, wastes that contain suspended total metals 3010 solids and mobility-procedure extracts aqueous samples, wastes that contain suspended total metals 3015 solids and mobility-procedure extracts aqueous samples, wastes that contain suspended total metals 3020 solids and mobility-procedure extracts total metals 3050 sediments, sludges and soil samples total metals 3051 sludges, sediment, soil and oil http://www.webapps.cee.vt.edu/ewr/environmental/teach/smprimer/aa/aa.html 11/8/2012 13
- 14. Sample Preparation ’é¦ Cara Basah ŌĆóTimbang 2,5 gr sampel, masukkan ke dalam gelas beker. Tambahkan 25 mL HNO3 pekat, tutup dengan gelas arloji, didihkan selama 30 ŌĆō 45 menit untuk mengoksidasi senyawa organik. Dinginkan larutan secara perlahan, tambahkan 10 mL HClO4 70%. Didihkan kembali hingga larutan menjadi jernih ’é¦ Cara Kering ŌĆóTimbang 2,5 gr sampel, masukkan ke dalam porselen. Panaskan dalam oven hingga suhu 550 oC selama 4 jam. Dinginkan, tambahkan 10 mL HCl 3 N. Tutup dengan gelas arloji, didihkan selama 10 menit. Dinginkan, saring dan masukkan ke dalam labu takar 100 mL, encerkan hingga batas dengan air bebas ion Sumber: Presentasi AAS, Ongki Arief Wisudawan & Akbar Ilham Manangkasi, Teknik Material ITB. 11/8/2012 14
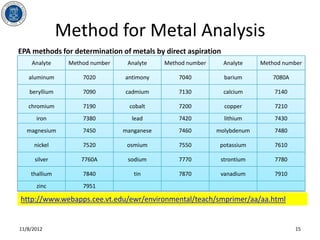
- 15. Method for Metal Analysis EPA methods for determination of metals by direct aspiration Analyte Method number Analyte Method number Analyte Method number aluminum 7020 antimony 7040 barium 7080A beryllium 7090 cadmium 7130 calcium 7140 chromium 7190 cobalt 7200 copper 7210 iron 7380 lead 7420 lithium 7430 magnesium 7450 manganese 7460 molybdenum 7480 nickel 7520 osmium 7550 potassium 7610 silver 7760A sodium 7770 strontium 7780 thallium 7840 tin 7870 vanadium 7910 zinc 7951 http://www.webapps.cee.vt.edu/ewr/environmental/teach/smprimer/aa/aa.html 11/8/2012 15
- 16. 7. Kalibrasi ŌĆó Interference check sample, ŌĆó Calibration verification, ŌĆó Calibration standards, ŌĆó Bland control, ŌĆó Linear dynamic range 11/8/2012 16
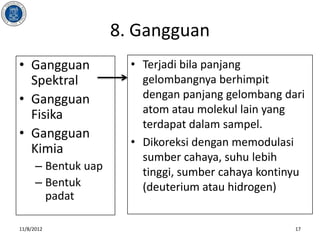
- 17. 8. Gangguan ŌĆó Gangguan ŌĆó Terjadi bila panjang Spektral gelombangnya berhimpit ŌĆó Gangguan dengan panjang gelombang dari Fisika atom atau molekul lain yang terdapat dalam sampel. ŌĆó Gangguan ŌĆó Dikoreksi dengan memodulasi Kimia sumber cahaya, suhu lebih ŌĆō Bentuk uap tinggi, sumber cahaya kontinyu ŌĆō Bentuk (deuterium atau hidrogen) padat 11/8/2012 17
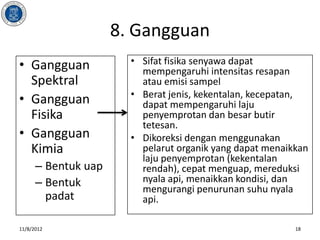
- 18. 8. Gangguan ŌĆó Gangguan ŌĆó Sifat fisika senyawa dapat mempengaruhi intensitas resapan Spektral atau emisi sampel ŌĆó Berat jenis, kekentalan, kecepatan, ŌĆó Gangguan dapat mempengaruhi laju Fisika penyemprotan dan besar butir tetesan. ŌĆó Gangguan ŌĆó Dikoreksi dengan menggunakan Kimia pelarut organik yang dapat menaikkan laju penyemprotan (kekentalan ŌĆō Bentuk uap rendah), cepat menguap, mereduksi ŌĆō Bentuk nyala api, menaikkan kondisi, dan mengurangi penurunan suhu nyala padat api. 11/8/2012 18
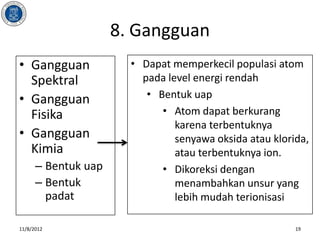
- 19. 8. Gangguan ŌĆó Gangguan ŌĆó Dapat memperkecil populasi atom Spektral pada level energi rendah ŌĆó Gangguan ŌĆó Bentuk uap Fisika ŌĆó Atom dapat berkurang karena terbentuknya ŌĆó Gangguan senyawa oksida atau klorida, Kimia atau terbentuknya ion. ŌĆō Bentuk uap ŌĆó Dikoreksi dengan ŌĆō Bentuk menambahkan unsur yang padat lebih mudah terionisasi 11/8/2012 19
- 20. 8. Gangguan ŌĆó Gangguan ŌĆó Bentuk padat ŌĆó Karena terbentuknya senyawa Spektral yang sukar menguap atau sukar ŌĆó Gangguan terdisosiasi dalam nyala api Fisika ŌĆó Terjadi ketika pelarut menguap dan meninggalkan partikel ŌĆó Gangguan padat. Kimia ŌĆó Dikoreksi dengan mengubah kondisi nyala, suhu lebih tinggi, ŌĆō Bentuk uap pemisahan secara selektif, ŌĆō Bentuk penambahan releasing agent, padat dan pengikatan unsur. 11/8/2012 20
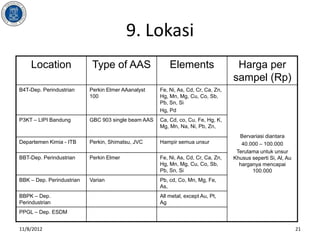
- 21. 9. Lokasi Location Type of AAS Elements Harga per sampel (Rp) B4T-Dep. Perindustrian Perkin Elmer AAanalyst Fe, Ni, As, Cd, Cr, Ca, Zn, 100 Hg, Mn, Mg, Cu, Co, Sb, Pb, Sn, Si Hg, Pd P3KT ŌĆō LIPI Bandung GBC 903 single beam AAS Ca, Cd, co, Cu, Fe, Hg, K, Mg, Mn, Na, Ni, Pb, Zn, Bervariasi diantara Departemen Kimia - ITB Perkin, Shimatsu, JVC Hampir semua unsur 40.000 ŌĆō 100.000 Terutama untuk unsur BBT-Dep. Perindustrian Perkin Elmer Fe, Ni, As, Cd, Cr, Ca, Zn, Khusus seperti Si, Al, Au Hg, Mn, Mg, Cu, Co, Sb, harganya mencapai Pb, Sn, Si 100.000 BBK ŌĆō Dep. Perindustrian Varian Pb, cd, Co, Mn, Mg, Fe, As, BBPK ŌĆō Dep. All metal, except Au, Pt, Perindustrian Ag PPGL ŌĆō Dep. ESDM 11/8/2012 21
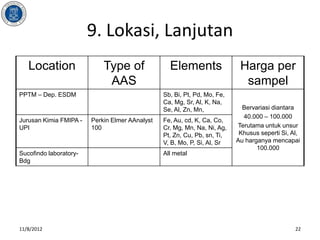
- 22. 9. Lokasi, Lanjutan Location Type of Elements Harga per AAS sampel PPTM ŌĆō Dep. ESDM Sb, Bi, Pt, Pd, Mo, Fe, Ca, Mg, Sr, Al, K, Na, Se, Al, Zn, Mn, Bervariasi diantara 40.000 ŌĆō 100.000 Jurusan Kimia FMIPA - Perkin Elmer AAnalyst Fe, Au, cd, K, Ca, Co, UPI 100 Cr, Mg, Mn, Na, Ni, Ag, Terutama untuk unsur Pt, Zn, Cu, Pb, sn, Ti, Khusus seperti Si, Al, V, B, Mo, P, Si, Al, Sr Au harganya mencapai 100.000 Sucofindo laboratory- All metal Bdg 11/8/2012 22
- 23. 10. Metoda Lain ŌĆó Flame Emission Spectroscopy (FES) ŌĆó Inductively Coupled Plasma - Atomic Emission Spectrometry (ICP-AES) 11/8/2012 23
- 24. 11. Pustaka ŌĆó ASM Handbook Volume 10, Material Characterization ŌĆó http://www.webapps.cee.vt.edu/ewr/environmental/t each/smprimer/aa/aa.html ŌĆó Presentasi AAS, Ongki Arief Wisudawan & Akbar Ilham Manangkasi, Teknik Material ITB. ŌĆó http://www.files.chem.vt.edu/chem- ed/spec/atomic/aa.html ŌĆó http://www.civil.iitm.ac.in ŌĆó http://en.wikipedia.org/wiki/Atomic_absorption_spect roscopy 11/8/2012 24
Editor's Notes
- #5: AbsorptionMatter can capture╠²electromagnetic radiation╠²and convert the energy of a photon to internal energy. This process is called absorption. Energy is transferred from the radiation field to the absorbing species. We describe the energy change of the absorber as a transition or an excitation from a lower energy level to a higher energy level. Since the energy levels of matter are quantized, only light of energy that can cause transitions from one level to another will be absorbed.The type of excitation depends on the╠²wavelength╠²of the light. Electrons are promoted to higher orbitals by ultraviolet or visible light, vibrations are excited by infrared light, and rotations are excited by microwaves.Absorption╠²spectroscopy╠²is one way to study the╠²energy levels╠²of the atoms, molecules, and solids. An absorption spectrum is the absorption of light as a function of wavelength. The spectrum of an atom or molecule depends on its energy-level structure, making absorption spectra useful for identifying compounds.Measuring the concentration of an absorbing species in a sample is accomplished by applying the╠²Beer-Lambert Law.
- #9: A cathode lamp (1), is a stable light source, which is necessary to emit the sharp characteristic spectrum of the element to be determined. A different cathode lamp is needed for each element, although there are some lamps that can be used to determine three or four different elements if the cathode contains all of them. Each time a lamp is changed, proper alignment is needed in order to get as much light as possible through the flame, where the analyte is being atomized, and into the monochromator.The atom cell (2), is the part with two major functions: nebulization of sample solution into a fine aerosol solution, and dissociation of the analyte elements into free gaseous ground state form. Not all the analyte goes through the flame, part of it is disposed.As the sample passes through the flame, the beam of light passes through it into the monochromator (3). The monochromator isolates the specific spectrum line emitted by the light source through spectral dispersion, and focuses it upon a photomultiplier detector (4), whose function is to convert the light signal into an electrical signal.The processing of electrical signal is fulfilled by a signal amplifier (5). The signal could be displayed for readout (6), or further fed into a data station (7) for printout by the requested format.
- #10: Gases mixture flame (1800 ŌĆō 4500 ┬║ C): air-propane, air-acetylene etc. ; Atomic absorption spectrometry quantifies the absorption of ground state atoms in the gaseous state ; The atoms absorb ultraviolet or visible light and make transitions to higher electronic energy levels . The analyte concentration is determined from the amount of absorption.
- #11: Light sourceThe light source is usually a╠²hollow-cathode lamp╠²of the element that is being measured.╠²Lasers╠²are also used in research instruments. Since lasers are intense enough to excite atoms to higher energy levels, they allow AA and╠²atomic fluorescence╠²measurements in a single instrument. The disadvantage of these narrow-band light sources is that only one element is measurable at a time.AtomizerAA spectroscopy requires that the analyte atoms be in the gas phase. Ions or atoms in a sample must undergo desolvation and vaporization in a high-temperature source such as a flame or graphite furnace.
- #12: Flame AA uses a slot type burner to increase the path length, and therefore to increase the total absorbance (see╠²Beer-Lambert law). Sample solutions are usually aspirated with the gas flow into a nebulizing/mixing chamber to form small droplets before entering the flame.Flame AA can only analyze solutions
- #13: The graphite furnace has several advantages over a flame. It is a much more efficient atomizer than a flame and it can directly accept very small absolute quantities of sample. It also provides a reducing environment for easily oxidized elements. Samples are placed directly in the graphite furnace and the furnace is electrically heated in several steps to dry the sample, ash organic matter, and vaporize the analyte atoms.Graphite furnace AA can accept solutions, slurries, or solid samples.
- #14: Depending on the information required, total recoverable metals, dissolved metals, suspended metals, and total metals could be obtained from a certain environmental matrix. Table above lists the EPA method number for sample processing in terms of the environmental matrices and information required. For more detail information, readers could refer to EPA document SW-846 "Test methods for evaluating solid wastes".Appropriate acid digestion is employed in these methods. Hydrochloric acid digestion is not suitable for samples which will be analyzed by graphite furnace atomic absorption spectroscopy because it can cause interferences during furnace atomization.
- #16: Flame atomic absorption methods are referred to as direct aspiration determinations. They are normally completed as single element analyses and are relatively free of interelement spectral interferences. For some elements, the temperature or type of flame used is critical. If flame and analytical conditions are not properly used, chemical and ionization interferences can occur.Graphite furnace atomic absorption spectrometry replaces the flame with an electrically heated graphite furnace. The major advantage of this technique is that the detection limit can be extremely low. It is applicable for relatively clean samples, however, interferences could be a real problem. It is important for the analyst to establish a set of analytical protocol which is appropriate for the sample to be analyzed and for the information required. Table 3 lists the available method for different metal analysis provided in EPA manual SW-846.
- #18: A)╠²Spectral interferences are due to radiation overlapping that of the light source. The interference radiation may be an emission line of another element or compound, or general background radiation from the flame, solvent, or analytical sample. This usually occurs when using organic solvents, but can also happen when determining sodium with magnesium present, iron with copper or iron with nickel.B)╠²Formation of compounds that do not dissociate in the flame. The most common example is the formation of calcium and strontium phosphates.C)╠²Ionization of the analyte reduces the signal. This is commonly happens to barium, calcium, strontium, sodium and potassium.D)╠²Matrix interferences due to differences between surface tension and viscosity of test solutions and standards.
- #19: E)╠²Broadening of a spectral line, which can occur due to a number of factors. The most common linewidth broadening effects are:1. Doppler effectThis effect arises because atoms will have different components of velocity along the line of observation.2. Lorentz effectThis effect occurs as a result of the concentration of foreign atoms present in the environment of the emitting or absorbing atoms. The magnitude of the broadening varies with the pressure of the foreign gases and their physical properties.3. Quenching effectIn a low-pressure spectral source, quenching collision can occur in flames as the result of the presence of foreign gas molecules with vibrational levels very close to the excited state of the resonance line.4. Self absorption or self-reversal effectThe atoms of the same kind as that emitting radiation will absorb maximum radiation at the centre of the line than at the wings, resulting in the change of shape of the line as well as its intensity. This effect becomes serious if the vapour which is absorbing radiation is considerably cooler than that which is emitting radiation.
- #22: PerguruanTinggi Kimia IndustriDeperindag, Medan SUMUT, Telp : 061-4266383InstitutTeknologiSepuluhNopember Surabaya, Jurusan Kimia FMIPA.BBPOM JABAR BidangPengujian Jl. Pasteur Bandung 022-4200381BalaiBesarTekstil Bandung Telp. 08122030084InstitutTeknologi Bandung Lab. Kimia Jl. Ganesa No.10 BandungLembagaIlmuPengetahuan Indonesia PusatPenelitian Dan Pengembangan Kimia Terapan. (Jl. Sangkuriang no.21 Bandung ).PPTM Jl. Sudirman Bandung.Sucofindo Jl. SoekarnoHatta BandungUPI Jurusan Kimia Jl. Setia Budi Bandung.
- #24: 1. FESPropane-butane flame ( 2000 ŌĆō 3000 ┬║ C);Optical filter is used to monitor for the selected emission wavelength produced by the analyte;Suitable for elements with low excitation energy (Na, K, Li, Rb and Ca).2. ICP - AESAtomic emission spectroscopy measures the intensity of light emitted by atoms or ions of the elements of interest at specific wavelengths;Inductively Coupled Plasma spectrometers use emission spectroscopy to detect and quantify elements in a sample; ICP-AES uses the argon plasma (6000-10000┬║ C) for atomization and excitation of the sample atoms; ICP-AES determines approximately all of the elements except gases and some non-metals (C, N, F, O, H).