Bab6 persamaan kimia
- 1. 11 PERSAMAAN KIMIAPERSAMAAN KIMIA Menyetarakan Koefisien ReaksiMenyetarakan Koefisien Reaksi ’āś NN22 (g)(g) + H+ H22 (g)(g) ŌåÆ NHŌåÆ NH33 (g)(g) Secara langsung :Secara langsung : NN22 (g)(g) + 3H+ 3H22 (g)(g) ŌåÆ 2NHŌåÆ 2NH33 (g)(g) ’āś AlAl (s)(s) + O+ O22 (g)(g) ŌåÆ AlŌåÆ Al22OO33 (s)(s) Dimisalkan a, b, c, ŌĆ”ŌĆ”Dimisalkan a, b, c, ŌĆ”ŌĆ” aAlaAl (s)(s) + bO+ bO22 (g)(g) ŌåÆ cAlŌåÆ cAl22OO33 (s)(s) Al :Al : a = 2ca = 2c O :O : 2b = 3c2b = 3c
- 2. 2 Misal c = 1, maka :Misal c = 1, maka : aa = 2c = 2.1 = 2= 2c = 2.1 = 2 2b = 3c = 3.12b = 3c = 3.1 b = 3/2b = 3/2 Didapatkan :Didapatkan : a = 2a = 2 a = 4a = 4 b = 3/2b = 3/2 x2x2 b = 3b = 3 c = 1c = 1 c = 2c = 2 jadi :jadi : 4Al4Al (s)(s) + 3O+ 3O22 (g)(g) ŌåÆ 2AlŌåÆ 2Al22OO33 (s)(s)
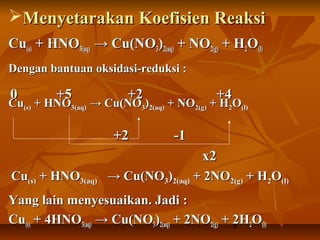
- 3. 3 ’āśMenyetarakan Koefisien ReaksiMenyetarakan Koefisien Reaksi CuCu(s)(s) + HNO+ HNO3(aq)3(aq) ŌåÆ Cu(NOŌåÆ Cu(NO33))2(aq)2(aq) + NO+ NO2(g)2(g) + H+ H22OO(l)(l) Dengan bantuan oksidasi-reduksi :Dengan bantuan oksidasi-reduksi : CuCu(s)(s) + HNO+ HNO3(aq)3(aq) ŌåÆ Cu(NOŌåÆ Cu(NO33))2(aq)2(aq) + NO+ NO2(g)2(g) + H+ H22OO(l)(l) CuCu(s)(s) + HNO+ HNO3(aq)3(aq) ŌåÆ Cu(NOŌåÆ Cu(NO33))2(aq)2(aq) + 2NO+ 2NO2(g)2(g) + H+ H22OO(l)(l) Yang lain menyesuaikan. Jadi :Yang lain menyesuaikan. Jadi : CuCu(s)(s) + 4HNO+ 4HNO3(aq)3(aq) ŌåÆ Cu(NOŌåÆ Cu(NO33))2(aq)2(aq) + 2NO+ 2NO2(g)2(g) + 2H+ 2H22OO(l)(l) 00 +5+5 +2+2 +4+4 +2+2 -1-1 x2x2
- 4. 4 Beberapa macam reaksi :Beberapa macam reaksi : ’ü« Reaksi Kombinasi/Penggabungan :Reaksi Kombinasi/Penggabungan : 4Al4Al (s)(s) + 3O+ 3O22 (g)(g) ŌåÆ 2AlŌåÆ 2Al22OO33 (s)(s) ’ü« Reaksi Penguraian :Reaksi Penguraian : Ca(HCOCa(HCO33))22 (s)(s) ŌåÆ CaOŌåÆ CaO (s)(s) + 2CO+ 2CO22 (g)(g) + H+ H22OO (l)(l) ’ü« Reaksi Substitusi/Pertukaran :Reaksi Substitusi/Pertukaran : ZnZn (s)(s) + 2HCl+ 2HCl (aq)(aq) ŌåÆ ZnClŌåÆ ZnCl22 (aq)(aq) + H+ H22 (g)(g) ’ü« Reaksi Netralisasi/Penggaraman :Reaksi Netralisasi/Penggaraman : AgNOAgNO33 (aq)(aq) + HCl+ HCl (aq)(aq) ŌåÆ AgClŌåÆ AgCl (s)(s) + HNO+ HNO33 (aq)(aq) HNOHNO33 (aq)(aq) + NaOH+ NaOH (aq)(aq) ŌåÆ NaNOŌåÆ NaNO33 (s)(s) + H+ H22OO (l)(l)