Chapter 3 matter
Download as PPT, PDF1 like1,524 views
The document discusses the classification and properties of matter. It defines the three physical states of matter as solid, liquid, and gas, which are determined by a substance's structure and ability to change shape. Pure substances are either elements or compounds, while mixtures can be either homogeneous or heterogeneous. Physical properties describe observable characteristics without changing composition, while chemical properties involve changes in composition through contact with other substances or energy. Phase changes that alter a substance's state, such as melting or boiling, are physical changes rather than chemical changes.
1 of 11
Downloaded 15 times










Recommended
Matter Notes



Matter Notesguestff6c3d1
?
Matter is anything that takes up space and has mass, including invisible substances. It is made of elements, the basic building blocks of atoms, and compounds, which are combinations of two or more elements chemically bonded together. Matter can also exist as mixtures of elements and compounds mixed together without chemical bonds. Physical changes alter the state of matter between solid, liquid and gas, while chemical changes create new substances through chemical reactions.Classification of matter and changes



Classification of matter and changesBhavana Goud
?
This document discusses the classification and properties of matter. It defines matter as anything that has mass and occupies space, and classifies matter based on its physical state as solid, liquid or gas. Solids have a fixed shape and volume, liquids take the shape of their container but maintain a fixed volume, and gases expand to fill their container. Matter can also be classified based on its composition - pure substances have a uniform composition, while mixtures are combinations of two or more substances. Elements are pure substances made of a single type of atom, while compounds are pure substances made of molecules containing two or more elements. The document distinguishes between physical changes, which alter a substance's state or properties without changing its composition, and chemical changes,Unit I: Matter



Unit I: MatterAdrianna Nading
?
This document discusses matter and its properties. It defines key terms like mass, matter, atom, element, compound, and describes properties of matter including intensive, extensive, and states of matter. It also classifies matter as mixtures (homogeneous, heterogeneous, solutions, colloids, suspensions) or pure substances. Finally, it distinguishes between physical and chemical properties/changes in matter.Keynote; ch. 2; nature of matter



Keynote; ch. 2; nature of matterwthompsonctems
?
All matter is made of atoms. Atoms of different elements have unique physical and chemical properties. Matter can be made of a single element or a combination of elements chemically bonded together to form compounds or mixtures. Compounds have different properties than their individual elements. Chemical and physical properties can be used to identify substances and chemical reactions which rearrange atoms but conserve total matter.Chem unit 2 presentation



Chem unit 2 presentationbobcatchemistry
?
This document provides an overview of chemistry unit 2 which covers how matter changes. It discusses the three common states of matter - solids, liquids and gases. It defines physical and chemical properties and changes. It also defines mixtures and how they are combinations of substances that can be separated. It introduces elements and compounds, explaining how compounds are combinations of elements that have different properties. It covers the laws of definite and multiple proportions for compounds.Chemistry - Chapter 2 matter and change



Chemistry - Chapter 2 matter and changeMr. Walajtys
?
This document provides an overview of chapter 2 which covers properties of matter, states of matter, mixtures, elements and compounds, and chemical reactions. It defines key terms including physical and chemical properties, extensive and intensive properties, elements, compounds, mixtures, homogeneous and heterogeneous mixtures, physical and chemical changes, reactants and products. It also outlines the key objectives covered in each section and provides examples to illustrate important concepts such as the three states of matter, separating mixtures, symbols and formulas of elements, and the law of conservation of mass.Properties Of Matter



Properties Of Matternoragzz
?
This document discusses the fundamental concepts of matter including atoms, elements, compounds, and mixtures. It defines chemistry as the study of matter and its properties. Properties can be physical, describing observable characteristics without changes in composition, or chemical, involving changes in what substances are present. Physical properties include things like color, texture, and boiling point while chemical properties relate to reactivity and changes in substances. The document also discusses the classification of matter as pure substances or mixtures and the differences between homogeneous and heterogeneous mixtures.Chemistry Unit 2 Part 1



Chemistry Unit 2 Part 1shawnschlueter
?
This document discusses different types of properties and changes in matter. It defines extensive and intensive properties, with examples like mass and density. Physical properties can be observed without changing a substance's identity, while chemical properties involve changes in a substance's composition or structure. Physical changes alter a substance's state or form without changing its chemical makeup, like phase changes between solid, liquid and gas. Chemical changes form new substances through chemical reactions. All changes require a transfer of energy.Chapter 3 Matter Properties And Changes



Chapter 3 Matter Properties And Changesfrhsd
?
This document provides an overview of matter and changes to matter. It defines key concepts such as elements, compounds, mixtures, physical and chemical properties, and states of matter. It also describes different types of physical and chemical changes, including phase changes, and explains techniques for separating mixtures like filtration, distillation, and crystallization. The document also introduces the concepts of energy and its various forms, and explains the laws of conservation of mass and energy.Ch 3 matter properties and changes



Ch 3 matter properties and changesEsther Herrera
?
This document summarizes key concepts about matter and its properties from a chemistry textbook chapter. It defines matter, mass, and weight. It describes the different states of matter and properties of substances and mixtures. It explains physical and chemical changes and how to classify them. It also outlines the concepts of elements, compounds, mixtures, and the periodic table.Chapter 2



Chapter 2SHERIFA s
?
This document provides an overview of key concepts about matter and changes in its state. It discusses extensive and intensive properties, and distinguishes between physical and chemical changes. Physical changes are reversible and do not alter composition, while chemical changes produce new substances. The document also introduces the concepts of elements, compounds, and mixtures, and describes different types of phase changes and chemical reactions.Chem Hapg1 Matter Properties And Changes



Chem Hapg1 Matter Properties And ChangesCesar Chavez HS
?
This document summarizes key concepts from a chapter on matter, including:
- Distinguishing between physical and chemical properties and changes
- Defining the three physical states of matter
- Explaining conservation of mass in chemical reactions
- Contrasting mixtures and pure substances
- Describing techniques to separate mixtures
- Distinguishing between elements and compounds
- Explaining the organization of the periodic table and laws of definite and multiple proportions governing compoundsChemical reactions



Chemical reactionsMrsKendall
?
This document summarizes key concepts about chemical reactions and properties. It defines chemical properties as the ability of a substance to change into a new substance, and physical properties as observable characteristics that do not change the substance. Chemical changes result in new substances, while physical changes alter appearance but not substance. For a chemical reaction to occur, bonds must be broken and reformed, allowing atoms to rearrange. Signs that a chemical reaction has taken place include formation of a precipitate, release of heat or light, and changes in color, temperature or gas formation. Chemical equations balance reactants and products to obey the law of conservation of matter, where mass and atoms are conserved despite rearrangement during bonding changes. Reaction rates depend on factors like activation energyMatter and its properties



Matter and its propertiesChris Mack
?
This document discusses the fundamental properties and classification of matter. It defines matter as anything that has mass and takes up space, and it is made of atoms which combine to form elements or compounds. The properties of matter can be extensive, depending on amount, or intensive, not depending on amount. Matter exists in solid, liquid, gas and plasma states and undergoes physical changes that do not alter its chemical identity or chemical changes that create new substances. Mixtures are combinations of substances that retain their own properties, while pure substances have consistent composition and properties regardless of sample.Characteristic properties of_matter



Characteristic properties of_matterMike Bryant
?
The document discusses the characteristic properties of matter. It states that matter has physical properties like color, odor, and melting point that can be observed without chemical changes. It also has chemical properties like acidity and reactivity that involve chemical changes. The characteristic properties do not depend on amount or shape of the matter. Some factors like heat, pressure, and nuclear processes can change the properties.Properties of matter



Properties of matterMuneeba Fatima
?
This document defines matter and discusses its properties and changes. It begins by defining matter as anything that occupies space and has mass. Matter's properties are then divided into physical and chemical. Physical properties can be measured without changing the substance's chemical identity, while chemical properties deal with how substances react with one another.
The document goes on to categorize physical properties as either intensive, meaning they do not depend on amount of matter present, or extensive, meaning they do depend on amount. Examples of intensive and extensive properties are given. The three states of matter - solid, liquid, and gas - are described. Physical and chemical changes are then defined, with a chemical change altering the composition of matter.Matter and its properties 



Matter and its properties Faraz Ahmed
?
Matter is anything that has mass and takes up space. There are three states of matter: solids have a definite shape and volume with particles close together and fixed; liquids have an indefinite shape but definite volume, taking the shape of their container with mobile but close particles; gases have an indefinite shape and volume, taking the shape and volume of their container with particles far apart and moving. Properties of matter include physical properties which are observed without changing the substance like color, shape, or boiling point, and chemical properties which are only observed when a substance interacts with another like flammability or rusting. Basic kinds of matter are elements, compounds, and mixtures.Good Presentation



Good Presentationsspurlock
?
The document discusses various topics related to matter and chemical changes. It defines elements and compounds, explains that compounds can undergo chemical changes while elements cannot, and states that the law of conservation of mass means the mass of reactants equals the mass of products in a chemical reaction. It also distinguishes physical and chemical changes, noting that only chemical changes alter the composition of matter.Chem change



Chem changeMrsKendall
?
This document discusses chemical and physical changes in matter. It defines chemical properties as those that describe a substance's ability to change into a different substance through a chemical reaction. Physical properties can be observed without a chemical change. Chemical changes result in new substances, while physical changes only alter the form or appearance. Evidence of a chemical change includes a change in properties or energy, with energy either absorbed or released. Chemical equations are used to represent chemical reactions and their reactants and products. The mass of reactants and products is conserved in any chemical change.Classification of matter e



Classification of matter eNatasia Gouws
?
The document defines key concepts in chemistry including physical and chemical properties of matter, types of mixtures and solutions, separation techniques like chromatography, and characteristics of pure substances and impure mixtures. It provides examples to illustrate concepts like homogeneous and heterogeneous mixtures, emulsions and suspensions, and explains processes like distillation and melting point determination that are used to separate or identify unknown substances.Chem chapt 3



Chem chapt 3bobcatchemistry
?
This document summarizes key concepts from a chemistry chapter about matter, its properties, and changes. It discusses the three common states of matter, physical and chemical properties, and physical and chemical changes. It also covers mixtures and their separation techniques, elements and compounds, and the laws of definite and multiple proportions.The Matter



The Matterjacobo durango echeverri
?
This document provides an overview of chemistry, including what it is, different types of chemistry, the scientific method, matter and its properties, energy, and chemical reactions. It defines chemistry as the study of matter, its composition and properties. It describes the major branches of chemistry and explains key concepts like the scientific method, states of matter, physical and chemical properties, and the conservation of mass and energy in chemical changes.Science šÝšÝßĢshow



Science šÝšÝßĢshowJordynAGraland
?
This document provides an overview of key chemistry concepts related to classifying and identifying matter. It defines pure substances, elements, compounds, mixtures and their subcategories. Physical properties like viscosity, conductivity and melting point are described, as well as common separation techniques like filtration and distillation. Chemical properties like reactivity and flammability are also covered. Key differences between physical and chemical changes are outlined. The document concludes with review questions to test understanding of these fundamental chemistry concepts.Matter Notes



Matter NotesMr. Brittle
?
Matter is anything that takes up space and has mass, including invisible substances. It is made of elements, the basic building blocks of atoms, and compounds, which are combinations of two or more elements chemically bonded together. Matter can also exist as mixtures of elements and compounds mixed together without chemical bonds. Physical changes alter the state of matter between solid, liquid and gas, while chemical changes create new substances through chemical reactions.Describing matter powerpoint



Describing matter powerpointkristannsnyder
?
Matter can exist in three states - solid, liquid, and gas. Examples of solids are ice and diamonds; examples of liquids are water and mercury. Gases include water vapor and oxygen. Characteristic properties like boiling point and melting point are used to identify substances and do not change, even if the substance changes state. Physical changes alter the substance's form through processes like melting or crushing, but do not change its chemical makeup. Chemical changes form new substances through chemical reactions.Minooka - Matter



Minooka - MatterJeanne Erfft
?
There are three main types of matter: pure substances, mixtures, and compounds. Pure substances are either elements or compounds that have consistent properties throughout. Compounds are formed by two or more elements chemically bonded together in fixed proportions, and have different properties than the constituent elements. Mixtures contain two or more substances mixed together without chemical bonding, and the substances retain their original properties and can be separated using physical means.Classification of Matter



Classification of MatterPaul Schumann
?
The document defines matter and describes its three common states: solid, liquid, and gas. It distinguishes between physical and chemical properties of matter, and between physical and chemical changes. It defines mixtures and pure substances, and classifies matter as homogeneous or heterogeneous. Key terms include the various states of matter, physical and chemical properties, and the classification of matter.Matter and change



Matter and changebriansitz
?
The document discusses the key properties and characteristics of matter. It defines matter as anything that has mass and takes up space. It describes the three main states of matter - solids, liquids, and gases - and their properties. It also discusses physical and chemical properties, mixtures and compounds, and how chemical and physical changes can alter matter.Chem unit 2 presentation



Chem unit 2 presentationbobcatchemistry
?
This document provides an overview of chemistry unit 2 which covers how matter changes. It discusses the three common states of matter, physical and chemical properties, and how matter undergoes physical and chemical changes. It also addresses mixtures as combinations of substances and how mixtures can be separated. The document defines elements and compounds, and explains the organization of elements in the periodic table. It describes how all compounds obey the laws of definite and multiple proportions.More Related Content
What's hot (14)
Chapter 3 Matter Properties And Changes



Chapter 3 Matter Properties And Changesfrhsd
?
This document provides an overview of matter and changes to matter. It defines key concepts such as elements, compounds, mixtures, physical and chemical properties, and states of matter. It also describes different types of physical and chemical changes, including phase changes, and explains techniques for separating mixtures like filtration, distillation, and crystallization. The document also introduces the concepts of energy and its various forms, and explains the laws of conservation of mass and energy.Ch 3 matter properties and changes



Ch 3 matter properties and changesEsther Herrera
?
This document summarizes key concepts about matter and its properties from a chemistry textbook chapter. It defines matter, mass, and weight. It describes the different states of matter and properties of substances and mixtures. It explains physical and chemical changes and how to classify them. It also outlines the concepts of elements, compounds, mixtures, and the periodic table.Chapter 2



Chapter 2SHERIFA s
?
This document provides an overview of key concepts about matter and changes in its state. It discusses extensive and intensive properties, and distinguishes between physical and chemical changes. Physical changes are reversible and do not alter composition, while chemical changes produce new substances. The document also introduces the concepts of elements, compounds, and mixtures, and describes different types of phase changes and chemical reactions.Chem Hapg1 Matter Properties And Changes



Chem Hapg1 Matter Properties And ChangesCesar Chavez HS
?
This document summarizes key concepts from a chapter on matter, including:
- Distinguishing between physical and chemical properties and changes
- Defining the three physical states of matter
- Explaining conservation of mass in chemical reactions
- Contrasting mixtures and pure substances
- Describing techniques to separate mixtures
- Distinguishing between elements and compounds
- Explaining the organization of the periodic table and laws of definite and multiple proportions governing compoundsChemical reactions



Chemical reactionsMrsKendall
?
This document summarizes key concepts about chemical reactions and properties. It defines chemical properties as the ability of a substance to change into a new substance, and physical properties as observable characteristics that do not change the substance. Chemical changes result in new substances, while physical changes alter appearance but not substance. For a chemical reaction to occur, bonds must be broken and reformed, allowing atoms to rearrange. Signs that a chemical reaction has taken place include formation of a precipitate, release of heat or light, and changes in color, temperature or gas formation. Chemical equations balance reactants and products to obey the law of conservation of matter, where mass and atoms are conserved despite rearrangement during bonding changes. Reaction rates depend on factors like activation energyMatter and its properties



Matter and its propertiesChris Mack
?
This document discusses the fundamental properties and classification of matter. It defines matter as anything that has mass and takes up space, and it is made of atoms which combine to form elements or compounds. The properties of matter can be extensive, depending on amount, or intensive, not depending on amount. Matter exists in solid, liquid, gas and plasma states and undergoes physical changes that do not alter its chemical identity or chemical changes that create new substances. Mixtures are combinations of substances that retain their own properties, while pure substances have consistent composition and properties regardless of sample.Characteristic properties of_matter



Characteristic properties of_matterMike Bryant
?
The document discusses the characteristic properties of matter. It states that matter has physical properties like color, odor, and melting point that can be observed without chemical changes. It also has chemical properties like acidity and reactivity that involve chemical changes. The characteristic properties do not depend on amount or shape of the matter. Some factors like heat, pressure, and nuclear processes can change the properties.Properties of matter



Properties of matterMuneeba Fatima
?
This document defines matter and discusses its properties and changes. It begins by defining matter as anything that occupies space and has mass. Matter's properties are then divided into physical and chemical. Physical properties can be measured without changing the substance's chemical identity, while chemical properties deal with how substances react with one another.
The document goes on to categorize physical properties as either intensive, meaning they do not depend on amount of matter present, or extensive, meaning they do depend on amount. Examples of intensive and extensive properties are given. The three states of matter - solid, liquid, and gas - are described. Physical and chemical changes are then defined, with a chemical change altering the composition of matter.Matter and its properties 



Matter and its properties Faraz Ahmed
?
Matter is anything that has mass and takes up space. There are three states of matter: solids have a definite shape and volume with particles close together and fixed; liquids have an indefinite shape but definite volume, taking the shape of their container with mobile but close particles; gases have an indefinite shape and volume, taking the shape and volume of their container with particles far apart and moving. Properties of matter include physical properties which are observed without changing the substance like color, shape, or boiling point, and chemical properties which are only observed when a substance interacts with another like flammability or rusting. Basic kinds of matter are elements, compounds, and mixtures.Good Presentation



Good Presentationsspurlock
?
The document discusses various topics related to matter and chemical changes. It defines elements and compounds, explains that compounds can undergo chemical changes while elements cannot, and states that the law of conservation of mass means the mass of reactants equals the mass of products in a chemical reaction. It also distinguishes physical and chemical changes, noting that only chemical changes alter the composition of matter.Chem change



Chem changeMrsKendall
?
This document discusses chemical and physical changes in matter. It defines chemical properties as those that describe a substance's ability to change into a different substance through a chemical reaction. Physical properties can be observed without a chemical change. Chemical changes result in new substances, while physical changes only alter the form or appearance. Evidence of a chemical change includes a change in properties or energy, with energy either absorbed or released. Chemical equations are used to represent chemical reactions and their reactants and products. The mass of reactants and products is conserved in any chemical change.Classification of matter e



Classification of matter eNatasia Gouws
?
The document defines key concepts in chemistry including physical and chemical properties of matter, types of mixtures and solutions, separation techniques like chromatography, and characteristics of pure substances and impure mixtures. It provides examples to illustrate concepts like homogeneous and heterogeneous mixtures, emulsions and suspensions, and explains processes like distillation and melting point determination that are used to separate or identify unknown substances.Chem chapt 3



Chem chapt 3bobcatchemistry
?
This document summarizes key concepts from a chemistry chapter about matter, its properties, and changes. It discusses the three common states of matter, physical and chemical properties, and physical and chemical changes. It also covers mixtures and their separation techniques, elements and compounds, and the laws of definite and multiple proportions.The Matter



The Matterjacobo durango echeverri
?
This document provides an overview of chemistry, including what it is, different types of chemistry, the scientific method, matter and its properties, energy, and chemical reactions. It defines chemistry as the study of matter, its composition and properties. It describes the major branches of chemistry and explains key concepts like the scientific method, states of matter, physical and chemical properties, and the conservation of mass and energy in chemical changes.Similar to Chapter 3 matter (20)
Science šÝšÝßĢshow



Science šÝšÝßĢshowJordynAGraland
?
This document provides an overview of key chemistry concepts related to classifying and identifying matter. It defines pure substances, elements, compounds, mixtures and their subcategories. Physical properties like viscosity, conductivity and melting point are described, as well as common separation techniques like filtration and distillation. Chemical properties like reactivity and flammability are also covered. Key differences between physical and chemical changes are outlined. The document concludes with review questions to test understanding of these fundamental chemistry concepts.Matter Notes



Matter NotesMr. Brittle
?
Matter is anything that takes up space and has mass, including invisible substances. It is made of elements, the basic building blocks of atoms, and compounds, which are combinations of two or more elements chemically bonded together. Matter can also exist as mixtures of elements and compounds mixed together without chemical bonds. Physical changes alter the state of matter between solid, liquid and gas, while chemical changes create new substances through chemical reactions.Describing matter powerpoint



Describing matter powerpointkristannsnyder
?
Matter can exist in three states - solid, liquid, and gas. Examples of solids are ice and diamonds; examples of liquids are water and mercury. Gases include water vapor and oxygen. Characteristic properties like boiling point and melting point are used to identify substances and do not change, even if the substance changes state. Physical changes alter the substance's form through processes like melting or crushing, but do not change its chemical makeup. Chemical changes form new substances through chemical reactions.Minooka - Matter



Minooka - MatterJeanne Erfft
?
There are three main types of matter: pure substances, mixtures, and compounds. Pure substances are either elements or compounds that have consistent properties throughout. Compounds are formed by two or more elements chemically bonded together in fixed proportions, and have different properties than the constituent elements. Mixtures contain two or more substances mixed together without chemical bonding, and the substances retain their original properties and can be separated using physical means.Classification of Matter



Classification of MatterPaul Schumann
?
The document defines matter and describes its three common states: solid, liquid, and gas. It distinguishes between physical and chemical properties of matter, and between physical and chemical changes. It defines mixtures and pure substances, and classifies matter as homogeneous or heterogeneous. Key terms include the various states of matter, physical and chemical properties, and the classification of matter.Matter and change



Matter and changebriansitz
?
The document discusses the key properties and characteristics of matter. It defines matter as anything that has mass and takes up space. It describes the three main states of matter - solids, liquids, and gases - and their properties. It also discusses physical and chemical properties, mixtures and compounds, and how chemical and physical changes can alter matter.Chem unit 2 presentation



Chem unit 2 presentationbobcatchemistry
?
This document provides an overview of chemistry unit 2 which covers how matter changes. It discusses the three common states of matter, physical and chemical properties, and how matter undergoes physical and chemical changes. It also addresses mixtures as combinations of substances and how mixtures can be separated. The document defines elements and compounds, and explains the organization of elements in the periodic table. It describes how all compounds obey the laws of definite and multiple proportions.Chapter 2 matter and change



Chapter 2 matter and changemcnewbold
?
This chapter discusses the fundamental concepts of matter and chemical changes. It defines matter as anything that has mass and takes up space, and describes its three main states as solid, liquid, and gas. Properties of matter are classified as either intensive or extensive. Physical and chemical properties are distinguished. Mixtures and compounds are introduced, where mixtures maintain the properties of the individual components and compounds exhibit new properties. Elements are defined as pure substances made of only one type of atom that cannot be broken down further by chemical means. Chemical changes, in which the reactants are converted into products with different compositions, are described along with clues that indicate a chemical change has occurred. The law of conservation of mass, whereby the mass of the reactants equals the massStates of Matter



States of Matterzehnerm2
?
The document discusses the kinetic molecular theory and states of matter. It introduces the four basic states of matter - solids, liquids, gases, and plasma - and describes their characteristic properties like shape, volume, and particle motion. It also discusses the classification of matter into pure substances and mixtures, and defines properties as either intensive or extensive, and changes as either physical or chemical.Honor Chemistry 2



Honor Chemistry 2Marissa tate
?
This document provides an overview of key chemistry concepts including:
1) A substance has a uniform and definite composition, while a mixture's composition can vary. Substances are identified by their intensive properties.
2) Matter exists in solid, liquid, and gas states depending on how tightly or loosely particles are packed. Physical changes alter properties but not composition.
3) A chemical change produces new substances with different compositions through rearrangement of atoms. Chemical properties and reactions allow identification of substances.
4) Chemical formulas and symbols represent the composition of compounds and elements. The subscripts in formulas indicate the relative proportions of elements in a compound.Ch 1 Matter in Our Surroundings šÝšÝßĢ show 3.ppt



Ch 1 Matter in Our Surroundings šÝšÝßĢ show 3.pptRajveerKaushal1
?
- Matter exists in solid, liquid, gas, and plasma states and undergoes physical and chemical changes. Physical changes alter a substance's state or form without changing its chemical makeup, while chemical changes create new substances.
- Substances can be elements, compounds, or mixtures. Elements cannot be broken down further, while compounds have a fixed composition and can be decomposed into simpler substances through chemical changes. Mixtures are combinations of substances that are not chemically bonded and have variable compositions.
- Plasma, the fourth state of matter, consists of free-floating ions and electrons. It is created by applying energy to strip electrons from atoms and can be controlled using electric and magnetic fields. Plasma research aids in understanding2. PPT DOWNLOADED.ppt



2. PPT DOWNLOADED.pptvaishalilikhar1
?
The document discusses the four states of matter and physical and chemical changes that matter undergoes. It provides details on the kinetic molecular theory explanation for differences between solids, liquids, and gases. Physical changes alter a substance's state or form without changing its chemical makeup, while chemical changes create new substances. The document also discusses plasma as the fourth state of matter and its many applications in manufacturing, medicine, and waste processing.Science keynote chapter 2



Science keynote chapter 2matisonmgraland
?
This document discusses different types of matter and changes in matter. It defines key terms like elements, compounds, mixtures, solutions, suspensions, and colloids. It explains that a pure substance has a uniform composition while a mixture's composition can vary. Physical properties can be observed without changing a substance's composition, while chemical properties involve changes in composition. Common separation methods like filtration and distillation are also outlined. The document stresses that physical changes do not alter a substance's composition, while chemical changes produce new substances.Matter



MatterKayla Jones
?
Matter can exist in different states and undergo physical or chemical changes. Physical changes alter a substance's state without changing its chemical makeup, while chemical changes form new substances. Properties like density and melting point can be used to identify pure substances and distinguish them from mixtures of multiple components.Matter



Mattermsnancy
?
Matter exists in various states and undergoes physical and chemical changes. Physical changes alter a substance's state without changing its chemical makeup, while chemical changes form new substances. Substances have consistent compositions and properties, whereas mixtures are combinations of substances that can be separated. Common states of matter include solids, liquids, and gases.Ch 2 Pre Ap Matter



Ch 2 Pre Ap Matterkermis
?
Matter exists in various states including solid, liquid, and gas. Physical changes alter the state of matter without changing its chemical composition, while chemical changes form new substances. Properties such as density and melting point can be used to identify substances and determine if a change is physical or chemical.Chapter two powerpoint



Chapter two powerpointNinaBGraland
?
The document summarizes key concepts about matter, including:
- Pure substances are classified as either elements or compounds.
- Elements contain only one type of atom, while compounds are made of two or more simpler substances.
- Mixtures can be heterogeneous or homogeneous based on how evenly distributed their parts are.
- Physical properties describe observable characteristics without changing a substance's composition.
- Chemical properties involve changes in composition through chemical reactions.Chapter 3 chemistry



Chapter 3 chemistrywikiuser0015
?
This document defines key concepts about the three states of matter, physical and chemical properties, and phase changes. It also discusses mixtures, elements, compounds, and the laws of definite and multiple proportions. The periodic table is introduced as organizing all elements. Methods for separating heterogeneous mixtures include filtration, distillation, crystallization, sublimation, and chromatography.Bad Presentation



Bad Presentationsspurlock
?
This document provides an overview of chemistry, including what chemistry is, its branches and types of research. It defines matter and discusses its building blocks and states. Pure substances like elements and compounds are introduced and distinguished from mixtures. Key concepts covered include physical and chemical properties/changes, energy and its role in changes, and the periodic table of elements.Recently uploaded (20)
Early Adopter's Guide to AI Moderation (Preview)



Early Adopter's Guide to AI Moderation (Preview)nick896721
?
Early Adopter's Guide to AI Moderation preview by User Interviews.Understanding Traditional AI with Custom Vision & MuleSoft.pptx



Understanding Traditional AI with Custom Vision & MuleSoft.pptxshyamraj55
?
Understanding Traditional AI with Custom Vision & MuleSoft.pptx | ### šÝšÝßĢ Deck Description:
This presentation features Atul, a Senior Solution Architect at NTT DATA, sharing his journey into traditional AI using Azure's Custom Vision tool. He discusses how AI mimics human thinking and reasoning, differentiates between predictive and generative AI, and demonstrates a real-world use case. The session covers the step-by-step process of creating and training an AI model for image classification and object detectionĄŠspecifically, an ad display that adapts based on the viewer's gender. Atulavan highlights the ease of implementation without deep software or programming expertise. The presentation concludes with a Q&A session addressing technical and privacy concerns.DAO UTokyo 2025 DLT mass adoption case studies IBM Tsuyoshi Hirayama (Æ―É―Ōã)



DAO UTokyo 2025 DLT mass adoption case studies IBM Tsuyoshi Hirayama (Æ―É―Ōã)Tsuyoshi Hirayama
?
DAO UTokyo 2025
|ūĐīóŅ§ĮéóŅ§h ĨÖĨíĨÃĨŊĨÁĨ§Đ`ĨóŅÐūŋĨĪĨËĨ·ĨĒĨÆĨĢĨÖ
https://utbciii.com/2024/12/12/announcing-dao-utokyo-2025-conference/
Session 1 :DLT mass adoption
IBM Tsuyoshi Hirayama (Æ―É―Ōã)DevNexus - Building 10x Development Organizations.pdf



DevNexus - Building 10x Development Organizations.pdfJustin Reock
?
Developer Experience is Dead! Long Live Developer Experience!
In this keynote-style session, weĄŊll take a detailed, granular look at the barriers to productivity developers face today and modern approaches for removing them. 10x developers may be a myth, but 10x organizations are very real, as proven by the influential study performed in the 1980s, ĄŪThe Coding War Games.ĄŊ
Right now, here in early 2025, we seem to be experiencing YAPP (Yet Another Productivity Philosophy), and that philosophy is converging on developer experience. It seems that with every new method, we invent to deliver products, whether physical or virtual, we reinvent productivity philosophies to go alongside them.
But which of these approaches works? DORA? SPACE? DevEx? What should we invest in and create urgency behind today so we donĄŊt have the same discussion again in a decade?UiPath Automation Developer Associate Training Series 2025 - Session 2



UiPath Automation Developer Associate Training Series 2025 - Session 2DianaGray10
?
In session 2, we will introduce you to Data manipulation in UiPath Studio.
Topics covered:
Data Manipulation
What is Data Manipulation
Strings
Lists
Dictionaries
RegEx Builder
Date and Time
Required Self-Paced Learning for this session:
Data Manipulation with Strings in UiPath Studio (v2022.10) 2 modules - 1h 30m - https://academy.uipath.com/courses/data-manipulation-with-strings-in-studio
Data Manipulation with Lists and Dictionaries in UiPath Studio (v2022.10) 2 modules - 1h - https:/academy.uipath.com/courses/data-manipulation-with-lists-and-dictionaries-in-studio
Data Manipulation with Data Tables in UiPath Studio (v2022.10) 2 modules - 1h 30m - https:/academy.uipath.com/courses/data-manipulation-with-data-tables-in-studio
?? For any questions you may have, please use the dedicated Forum thread. You can tag the hosts and mentors directly and they will reply as soon as possible. Gojek Clone Multi-Service Super App.pptx



Gojek Clone Multi-Service Super App.pptxV3cube
?
Gojek Clone is a versatile multi-service super app that offers ride-hailing, food delivery, payment services, and more, providing a seamless experience for users and businesses alike on a single platform.Formal Methods: Whence and Whither? [Martin Fr?nzle Festkolloquium, 2025]![Formal Methods: Whence and Whither? [Martin Fr?nzle Festkolloquium, 2025]](https://cdn.slidesharecdn.com/ss_thumbnails/mf2025-250305164811-a0930761-thumbnail.jpg?width=560&fit=bounds)
![Formal Methods: Whence and Whither? [Martin Fr?nzle Festkolloquium, 2025]](https://cdn.slidesharecdn.com/ss_thumbnails/mf2025-250305164811-a0930761-thumbnail.jpg?width=560&fit=bounds)
![Formal Methods: Whence and Whither? [Martin Fr?nzle Festkolloquium, 2025]](https://cdn.slidesharecdn.com/ss_thumbnails/mf2025-250305164811-a0930761-thumbnail.jpg?width=560&fit=bounds)
![Formal Methods: Whence and Whither? [Martin Fr?nzle Festkolloquium, 2025]](https://cdn.slidesharecdn.com/ss_thumbnails/mf2025-250305164811-a0930761-thumbnail.jpg?width=560&fit=bounds)
Formal Methods: Whence and Whither? [Martin Fr?nzle Festkolloquium, 2025]Jonathan Bowen
?
Alan Turing arguably wrote the first paper on formal methods 75 years ago. Since then, there have been claims and counterclaims about formal methods. Tool development has been slow but aided by MooreĄŊs Law with the increasing power of computers. Although formal methods are not widespread in practical usage at a heavyweight level, their influence as crept into software engineering practice to the extent that they are no longer necessarily called formal methods in their use. In addition, in areas where safety and security are important, with the increasing use of computers in such applications, formal methods are a viable way to improve the reliability of such software-based systems. Their use in hardware where a mistake can be very costly is also important. This talk explores the journey of formal methods to the present day and speculates on future directions.
Inside Freshworks' Migration from Cassandra to ScyllaDB by Premkumar Patturaj



Inside Freshworks' Migration from Cassandra to ScyllaDB by Premkumar PatturajScyllaDB
?
Freshworks migrated from Cassandra to ScyllaDB to handle growing audit log data efficiently. Cassandra required frequent scaling, complex repairs, and had non-linear scaling. ScyllaDB reduced costs with fewer machines and improved operations. Using Zero Downtime Migration (ZDM), they bulk-migrated data, performed dual writes, and validated consistency.30B Images and Counting: Scaling Canva's Content-Understanding Pipelines by K...



30B Images and Counting: Scaling Canva's Content-Understanding Pipelines by K...ScyllaDB
?
Scaling content understanding for billions of images is no easy feat. This talk dives into building extreme label classification models, balancing accuracy & speed, and optimizing ML pipelines for scale. You'll learn new ways to tackle real-time performance challenges in massive data environments.Cloud of everything Tech of the 21 century in Aviation



Cloud of everything Tech of the 21 century in AviationAssem mousa
?
AI, Block chain, Digital Currency, Cloud, Cloud of Things, Tactile Internet, Digital Twins, IOT, AR, VR, MR, U commerce, data and robotics."
Transform Your Future with Front-End Development Training



Transform Your Future with Front-End Development TrainingVtechlabs
?
Kickstart your career in web development with our front-end web development course in Vadodara. Learn HTML, CSS, JavaScript, React, and more through hands-on projects and expert mentorship. Our front-end development course with placement includes real-world training, mock interviews, and job assistance to help you secure top roles like Front-End Developer, UI/UX Developer, and Web Designer.
Join VtechLabs today and build a successful career in the booming IT industry!DealBook of Ukraine: 2025 edition | AVentures Capital



DealBook of Ukraine: 2025 edition | AVentures CapitalYevgen Sysoyev
?
The DealBook is our annual overview of the Ukrainian tech investment industry. This edition comprehensively covers the full year 2024 and the first deals of 2025. Fl studio crack version 12.9 Free Download



Fl studio crack version 12.9 Free Downloadkherorpacca127
?
https://ncracked.com/7961-2/
Note: >>?? Please copy the link and paste it into Google New Tab now Download link
The ultimate guide to FL Studio 12.9 Crack, the revolutionary digital audio workstation that empowers musicians and producers of all levels. This software has become a cornerstone in the music industry, offering unparalleled creative capabilities, cutting-edge features, and an intuitive workflow.
With FL Studio 12.9 Crack, you gain access to a vast arsenal of instruments, effects, and plugins, seamlessly integrated into a user-friendly interface. Its signature Piano Roll Editor provides an exceptional level of musical expression, while the advanced automation features empower you to create complex and dynamic compositions.Endpoint Backup: 3 Reasons MSPs Ignore It



Endpoint Backup: 3 Reasons MSPs Ignore ItMSP360
?
Many MSPs overlook endpoint backup, missing out on additional profit and leaving a gap that puts client data at risk.
Join our webinar as we break down the top challenges of endpoint backupĄŠand how to overcome them.MIND Revenue Release Quarter 4 2024 - Finacial Presentation



MIND Revenue Release Quarter 4 2024 - Finacial PresentationMIND CTI
?
MIND Revenue Release Quarter 4 2024 - Finacial PresentationUnlocking DevOps Secuirty :Vault & Keylock



Unlocking DevOps Secuirty :Vault & KeylockHusseinMalikMammadli
?
DevOps i? t?hlĻđk?sizliyi sizi maraqland?r?r? ?st?r developer, ist?r t?hlĻđk?sizlik mĻđh?ndisi, ist?rs? d? DevOps h?v?skar? olun, bu t?dbir ??b?k?l??m?k, bilikl?rinizi b?lĻđ?m?k v? DevSecOps sah?sind? ?n son t?crĻđb?l?ri ?yr?nm?k Ļđ?Ļđn mĻđk?mm?l fĻđrs?tdir!
Bu workshopda DevOps infrastrukturlar?n?n t?hlĻđk?sizliyini nec? art?rmaq bar?d? dan??acay?q. DevOps sisteml?ri qurulark?n avtomatla?d?r?lm??, yĻđks?k ?l?atan v? etibarl? olmas? il? yana??, h?m d? t?hlĻđk?sizlik m?s?l?l?ri n?z?r? al?nmal?d?r. Bu s?b?bd?n, DevOps komandolar?n?n t?hlĻđk?sizliy? y?n?lmi? praktikalara riay?t etm?si vacibdir.AIXMOOC 2.3 - Modelli di reti neurali con esperimenti di addestramento



AIXMOOC 2.3 - Modelli di reti neurali con esperimenti di addestramentoAlessandro Bogliolo
?
Lezione tenuta da Alessandro Bogliolo nell'ambito del MOOC dell'UniversitĻĪ di Urbino dedicato a LLMs e IA generativa
https://mooc.uniurb.it/aixmooc A Framework for Model-Driven Digital Twin Engineering



A Framework for Model-Driven Digital Twin EngineeringDaniel Lehner
?
šÝšÝßĢs from my PhD Defense at Johannes Kepler University, held on Janurary 10, 2025.
The full thesis is available here: https://epub.jku.at/urn/urn:nbn:at:at-ubl:1-83896Replacing RocksDB with ScyllaDB in Kafka Streams by Almog Gavra



Replacing RocksDB with ScyllaDB in Kafka Streams by Almog GavraScyllaDB
?
Learn how Responsive replaced embedded RocksDB with ScyllaDB in Kafka Streams, simplifying the architecture and unlocking massive availability and scale. The talk covers unbundling stream processors, key ScyllaDB features tested, and lessons learned from the transition.UiPath Agentic Automation Capabilities and Opportunities



UiPath Agentic Automation Capabilities and OpportunitiesDianaGray10
?
Learn what UiPath Agentic Automation capabilities are and how you can empower your agents with dynamic decision making. In this session we will cover these topics:
What do we mean by Agents
Components of Agents
Agentic Automation capabilities
What Agentic automation delivers and AI Tools
Identifying Agent opportunities
? If you have any questions or feedback, please refer to the "Women in Automation 2025" dedicated Forum thread. You can find there extra details and updates.Chapter 3 matter
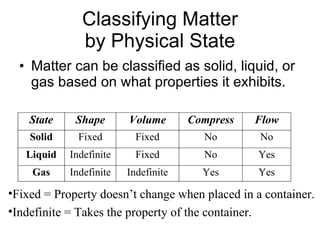
- 1. Classifying Matter by Physical State Matter can be classified as solid, liquid, or gas based on what properties it exhibits. Fixed = Property doesnĄŊt change when placed in a container. Indefinite = Takes the property of the container.
- 2. Structure Determines Properties The atoms or molecules have different structures in solids, liquids, and gases ? leading to different properties.
- 3. Classification of Matter by Composition Pure Substance = All samples are made of the same pieces in the same percentages. Salt Mixtures = Different samples may have the same pieces in different percentages. Salt water Pure Substance Constant Composition Homogeneous Mixture Variable Composition Matter
- 4. Classification of Pure Substances 1. Made of one type of atom. (Some elements are found as multi-atom molecules in nature.) 2. Combine together to make compounds. 1. Made of one type of molecule, or array of ions. 2. Molecules contain 2 or more different kinds of atoms. Elements Compounds
- 5. Classification of Mixtures 1. Made of multiple substances, but appears to be one substance. 2. All portions of a sample have the same composition and properties. 1. Made of multiple substances, whose presence can be seen. 2. Portions of a sample have different composition and properties. Heterogeneous Homogeneous
- 7. Properties of Matter Physical Properties are the characteristics of matter that can be changed without changing its composition. Characteristics that are directly observable. Chemical Properties are the characteristics that determine how the composition of matter changes as a result of contact with other matter or the influence of energy. Characteristics that describe the behavior of matter.
- 10. Changes in Matter Changes that alter the state or appearance of the matter without altering the composition are called physical changes . Changes that alter the composition of the matter are called chemical changes . During the chemical change, the atoms that are present rearrange into new molecules, but all of the original atoms are still present.
- 11. Phase Changes Are Physical Changes Boiling = liquid to gas. Melting = solid to liquid. Subliming = solid to gas. Freezing = liquid to solid. Condensing = gas to liquid. Deposition = gas to solid. State changes require heating or cooling the substance. Evaporation is not a simple phase change, it is a solution process.
