Density Functional Theory (DFT) Overview.pptx
Density Functional Theory (DFT) is a powerful computational method used to study the electronic structure of molecules and materials by focusing on electron density rather than the many-body wave function. DFT is preferred due to its efficiency, accuracy, and versatility, making it applicable in diverse fields like material design, catalysis, and drug discovery. When applied to the HŌééO molecule, DFT accurately predicts its molecular geometry, bond angles, and bond lengths, and provides insights into its electron density distribution, which reveals its polar nature. DFT simplifies the study of many-particle systems by reducing the problem to a manageable form, allowing for efficient calculations of large systems. The Born-Oppenheimer approximation further simplifies DFT by treating nuclear and electronic motions separately, significantly reducing the computational cost. At its core, the Hohenberg-Kohn theorem provides the theoretical foundation of DFT, stating that all properties of a quantum system can be determined by its electron density, making it a cornerstone of modern computational chemistry and materials science. In this PPT, we have explained the fundamentals of Density Functional Theory (DFT), its importance, and its application to the HŌééO molecule, along with key concepts like electron density, many-particle systems, the Born-Oppenheimer approximation, and the Hohenberg-Kohn theorem, highlighting its relevance in modern computational chemistry.







![Hohenberg-kohn Theorem 1
Ō¢¬ The Ground state energy E is the unique functional of
Electron Density
E = E ŃĆé [po(r)]
Ō¢¬ where p (r) represents the density function which itself is a
function of position (r).](https://image.slidesharecdn.com/densityfunctionaltheoryoverview-241215172219-46ba724c/85/Density-Functional-Theory-DFT-Overview-pptx-21-320.jpg)
![Ō¢¬ The electron density that minimizes the energy of the
overall functional is the true ground state electron density:
E[p(r)]>E.[p.(r)]
Hohenberg-kohn Theorem 2](https://image.slidesharecdn.com/densityfunctionaltheoryoverview-241215172219-46ba724c/85/Density-Functional-Theory-DFT-Overview-pptx-22-320.jpg)




Recommended




















































More Related Content
Similar to Density Functional Theory (DFT) Overview.pptx (20)








































More from momnaqayyum01 (9)


















Recently uploaded (20)






































Density Functional Theory (DFT) Overview.pptx
- 1. DENSITY FUNCTIONAL THEORY MOMNA QAYYUM Department of Chemistry University of Management and Technology, Lahore
- 2. CONTENTS Ō¢¬ DFT Ō¢¬ Why DFT Ō¢¬ H 2O Molecule Ō¢¬ Electron Density Ō¢¬ Many Particle System Ō¢¬ Born-Oppenheimer Approximations Ō¢¬ Hohenburg Kohn Theorem Ō¢¬ References
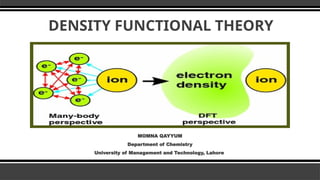
- 3. Density Functional Theory Density functional theory (DFT) is a quantum-mechanical atomistic simulation method to compute a wide variety of properties of almost any kind of atomic system: molecules, crystals, surfaces, and even electronic devices when combined with non-equilibrium Green's functions (NEGF).
- 5. Why DFT Schrodinger Equation can be solved easily for one electron system. For multiple electronic system, it is very hard to solve the Schrodinger equation. Ō¢¬ So we introduce some Approximations, one of them is DFT Ō¢¬ Reduce electron-electron interactions.
- 6. H20 Molecule
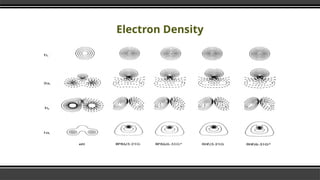
- 7. HŌééO Molecule Explained by DFT Ō¢¬ Density Functional Theory (DFT): Ō¢¬ Models the behavior of electrons in the HŌééO molecule using electron density, not wave functions. Ō¢¬ Efficiently predict molecular geometry, bond strengths, and electronic properties. Ō¢¬ Electron Density Distribution: Ō¢¬ DFT calculates the 3D electron density, showing how electrons are distributed around the oxygen and hydrogen atoms. Ō¢¬ Highlights the polar nature of the HŌééO molecule, with a higher electron density near oxygen.
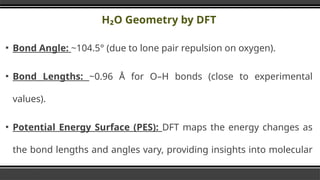
- 8. HŌééO Geometry by DFT Ō¢¬ Bond Angle: ~104.5┬░ (due to lone pair repulsion on oxygen). Ō¢¬ Bond Lengths: ~0.96 ├ģ for OŌĆōH bonds (close to experimental values). Ō¢¬ Potential Energy Surface (PES): DFT maps the energy changes as the bond lengths and angles vary, providing insights into molecular stability.
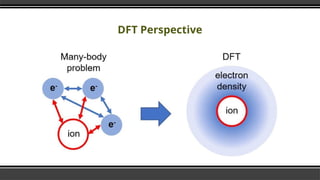
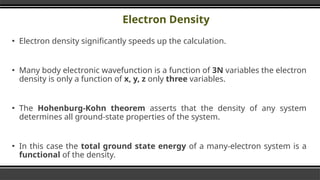
- 10. Electron Density Ō¢¬ Electron density significantly speeds up the calculation. Ō¢¬ Many body electronic wavefunction is a function of 3N variables the electron density is only a function of x, y, z only three variables. Ō¢¬ The Hohenburg-Kohn theorem asserts that the density of any system determines all ground-state properties of the system. Ō¢¬ In this case the total ground state energy of a many-electron system is a functional of the density.
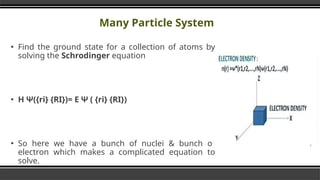
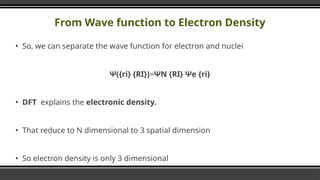
- 11. Many Particle System Ō¢¬ Find the ground state for a collection of atoms by solving the Schrodinger equation Ō¢¬ ╬Ś ╬©({ri} {RI})= ╬Ģ ╬© ( {ri} {RI}) Ō¢¬ So here we have a bunch of nuclei & bunch of electron which makes a complicated equation to solve.
- 12. Born-Oppenheimer Approximations Ō¢¬ According to this approximation: Ō¢¬ Mass of nuclei is very greater than mass of electrons N>>>>e Ō¢¬ Thus nuclei are slow and electrons are fast. Ō¢¬ In other words we can write the Schrodinger wave equation by separating the electronic and nuclei terms.
- 13. Key Concepts of Born-Oppenheimer Approximations 1. Separation of Motion: Ō¢¬ Assumes that nuclei are much heavier than electrons, so their motion can be treated separately. Ō¢¬ Electrons adjust instantly to the movement of nuclei. 2. Simplifies the Schr├Čdinger Equation: Ō¢¬ Treats the electronic and nuclear wavefunctions independently, drastically reducing computational complexity. 3. Energy Surface: Ō¢¬ The nuclei move on a potential energy surface defined by the electronic energy.
- 14. Why is This Important Ō¢¬ Simplifies Molecular Calculations: Reduces a complex multi- particle system into more manageable parts. Ō¢¬ Basis for Quantum Chemistry: Enables understanding of chemical bonding and molecular dynamics. Ō¢¬ Accurate Models: Provides realistic approximations for molecular structure and spectroscopy.
- 15. Practical Applications Ō¢¬ Molecular Vibrations: Explains vibrational spectra by treating nuclei separately. Ō¢¬ Chemical Reactions: Helps map potential energy surfaces to understand reaction pathways. Ō¢¬ Spectroscopy: Provides a framework for interpreting electronic, vibrational, and rotational spectra. Ō¢¬ Molecular Dynamics Simulations: Simplifies calculations for simulating large systems. Ō¢¬ Quantum Chemistry Software: Foundational principle behind tools like Gaussian and VASP.
- 16. Hohenberg-kohn Theorem Ō¢¬ The Ground state energy E is the unique functional of Electron Density This foundational concept in Density Functional Theory (DFT) has two key parts: 1. The Ground State Density Uniquely Defines All Properties Ō¢¬ The total energy and all properties of a quantum system can be determined entirely from the electron density, not the many-electron wave function. Ō¢¬ This simplifies complex quantum calculations by reducing dimensions. 2. Existence of a Universal Energy Functional Ō¢¬ There exists a mathematical function that relates the energy of the system to the electron density. Ō¢¬ The ground-state energy corresponds to the minimum of this energy functional.
- 17. Breaking Down the Hohenberg-kohn Theorem 1. Why Focus on Electron Density? Ō¢¬ Electron density tells us where electrons are most likely to be in a molecule or material. Ō¢¬ ItŌĆÖs much simpler than the many-body wave function but still contains all the essential information. 2. Implications of the Theorem Ō¢¬ The energy and behavior of a system can be calculated without solving the complex Schr├Čdinger equation directly. Ō¢¬ This reduces the problem's complexity from many-electron interactions to just density distribution.
- 18. Key Concepts of Hohenberg-kohn Theorem Ō¢¬ The ground-state electron density uniquely determines all properties of a quantum system (e.g., energy, reactivity). Ō¢¬ Reduces complexity: Depends on 3 spatial variables, not the 3N variables of the wavefunction (N = number of electrons). Ō¢¬ A mathematical function relates the energy of the system to the electron density. Ō¢¬ The ground-state energy corresponds to the minimum of this functional. Ō¢¬ The system is in its ground state (lowest energy). Ō¢¬ The relationship between energy and density is universal for all systems.
- 19. Why is This Important Ō¢¬ Simplifies Quantum Calculations: Reduces complexity from multi-electron wavefunctions to a 3-variable electron density. Ō¢¬ Foundation of DFT: Forms the basis for Density Functional Theory, a key tool in computational chemistry and physics. Ō¢¬ Reduces Computational Cost: Enables accurate predictions for large systems with minimal computational effort. Ō¢¬ Broad Applicability: Widely used in material science, catalysis, energy storage, and drug discovery. Ō¢¬ Revolutionized Modern Chemistry: Transformed the study and design of molecules and materials with efficient modeling techniques.
- 20. Practical Applications Ō¢¬ Material design: Predicts stability, conductivity, and magnetic properties of materials. Ō¢¬ Drug Discovery: Models molecular interactions with biological targets. Ō¢¬ Catalysis: Optimizes catalysts for faster and greener reactions. Ō¢¬ Battery Development: Designs more efficient energy storage materials. Ō¢¬ Environmental Science: Models pollutant behavior and chemical reactions in the atmosphere.
- 21. Hohenberg-kohn Theorem 1 Ō¢¬ The Ground state energy E is the unique functional of Electron Density E = E ŃĆé [po(r)] Ō¢¬ where p (r) represents the density function which itself is a function of position (r).
- 22. Ō¢¬ The electron density that minimizes the energy of the overall functional is the true ground state electron density: E[p(r)]>E.[p.(r)] Hohenberg-kohn Theorem 2
- 23. From Wave function to Electron Density Ō¢¬ So, we can separate the wave function for electron and nuclei ╬©({ri} {RI})=╬©N {RI} ╬©e {ri} Ō¢¬ DFT explains the electronic density. Ō¢¬ That reduce to N dimensional to 3 spatial dimension Ō¢¬ So electron density is only 3 dimensional
- 25. References Ō¢¬ Roienko, O., Lukin, V., Oliinyk, V., Djurovi─ć, I., & Simeunovi─ć, M. (2022). An Overview of the Adaptive Robust DFT and its Applications. Technological Innovation in Engineering Research, 4, 68-89. Ō¢¬ Chakraborty, D., & Chattaraj, P. K. (2021). Conceptual density functional theory based electronic structure principles. Chemical Science, 12(18), 6264-6279. Ō¢¬ Fiechter, M. R., & Richardson, J. O. (2024). Understanding the cavity BornŌĆōOppenheimer approximation. The Journal of Chemical Physics, 160(18). Ō¢¬ Liebert, J., & Schilling, C. (2023). An exact one-particle theory of bosonic excitations: from a generalized HohenbergŌĆōKohn theorem to convexified N-representability. New Journal of Physics, 25(1), 013009.
- 26. THANK YOU





